Question: Solutions to diffusion equation. a . Fick's First Law, Steady - State Diffusion. Purification of hydrogen from a gas mixture will be an important process
Solutions to diffusion equation.
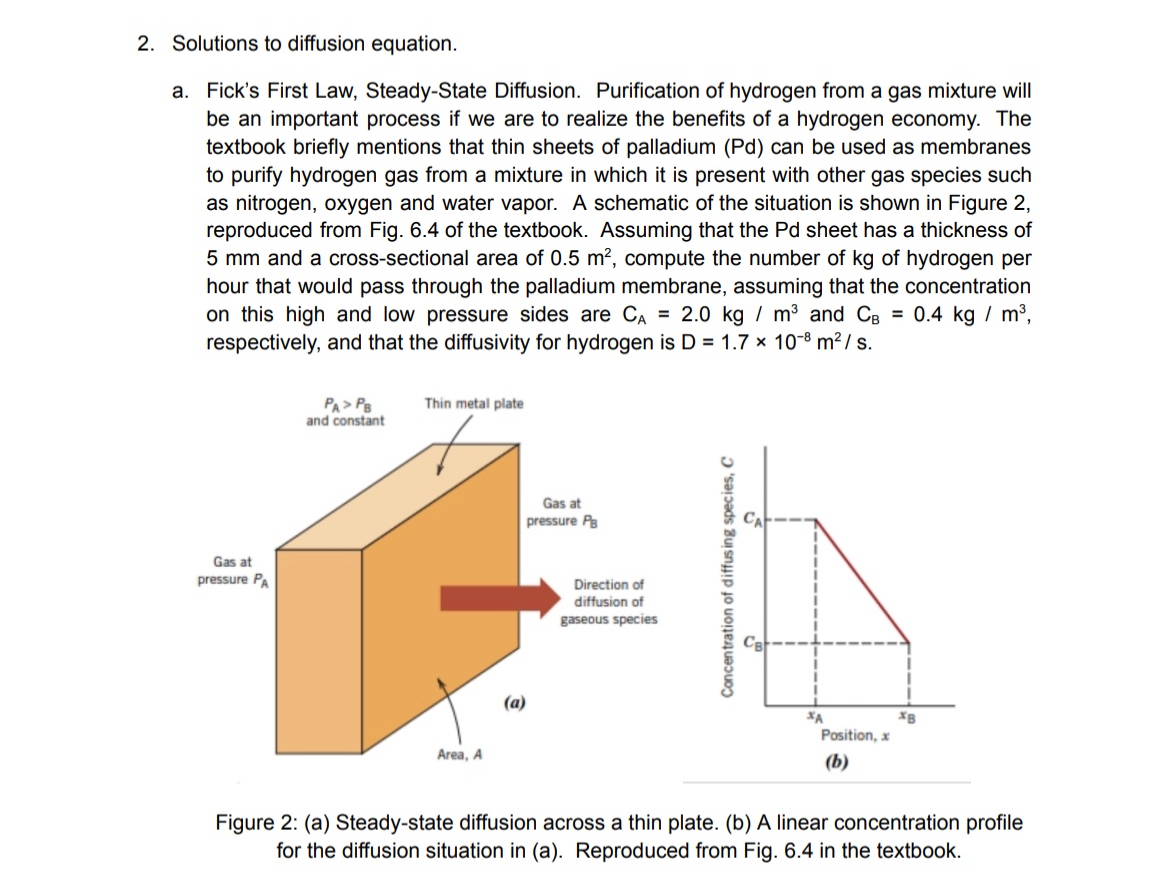
a Fick's First Law, SteadyState Diffusion. Purification of hydrogen from a gas mixture will be an important process if we are to realize the benefits of a hydrogen economy. The textbook briefly mentions that thin sheets of palladium Pd can be used as membranes to purify hydrogen gas from a mixture in which it is present with other gas species such as nitrogen, oxygen and water vapor. A schematic of the situation is shown in Figure reproduced from Fig. of the textbook. Assuming that the Pd sheet has a thickness of and a crosssectional area of compute the number of of hydrogen per hour that would pass through the palladium membrane, assuming that the concentration on this high and low pressure sides are and respectively, and that the diffusivity for hydrogen is
Figure : a Steadystate diffusion across a thin plate. b A linear concentration profile for the diffusion situation in a Reproduced from Fig. in the textbook.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


