Question: solve 2, 3 and plot graph for the data ty =m2V2A=7logt Experiment 35395 Plot the calibration curve of absorbance verus molar concentration for the standard
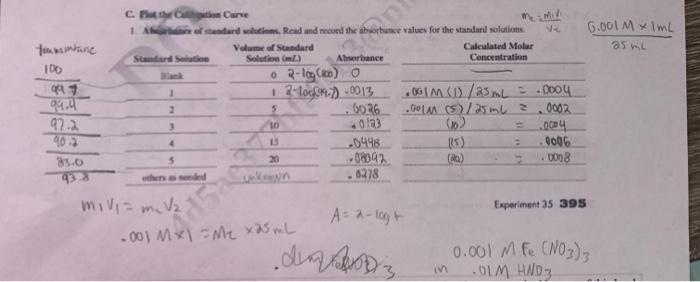

=m2V2A=7logt Experiment 35395 Plot the calibration curve of absorbance verus molar concentration for the standard solutions. Dato Anolysis, F,G Calculate the absarptivity coefficien for the metal ion. Have the instructor approve your graph. D. Unknown Metal Ion Concentration 1. Prepare the unknown. Describe the preparation for the solution containing the unknown concentration of the metal ion. Volume of unknown sample solution (mL) Polume of diluted sample (mL) 2. Concentration of metal ion. Absorbance of metal ion in solution Concentration of metal ion from the calibration curve (mol/L) Concentration of metal ion in the original sample corrected for dilution (moU/L) Show your calculations to account for the dilution of the original sample. 3. Expressing Concentration. Express the concentration of the metal ion in the sample in appropriate units of mass volume [ie., pph (pereent), ppm, ppb, ete.]. Concentration of metal ion in the original sample (massholume) Show calculations. =m2V2A=7logt Experiment 35395 Plot the calibration curve of absorbance verus molar concentration for the standard solutions. Dato Anolysis, F,G Calculate the absarptivity coefficien for the metal ion. Have the instructor approve your graph. D. Unknown Metal Ion Concentration 1. Prepare the unknown. Describe the preparation for the solution containing the unknown concentration of the metal ion. Volume of unknown sample solution (mL) Polume of diluted sample (mL) 2. Concentration of metal ion. Absorbance of metal ion in solution Concentration of metal ion from the calibration curve (mol/L) Concentration of metal ion in the original sample corrected for dilution (moU/L) Show your calculations to account for the dilution of the original sample. 3. Expressing Concentration. Express the concentration of the metal ion in the sample in appropriate units of mass volume [ie., pph (pereent), ppm, ppb, ete.]. Concentration of metal ion in the original sample (massholume) Show calculations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


