Question: Solve for acetone/methanol binary Table 13.10: Parameter Values for the Wilson and NRTL Equations Parameters a12,a21,b12, and b21 have units of cal. mol1, and V1
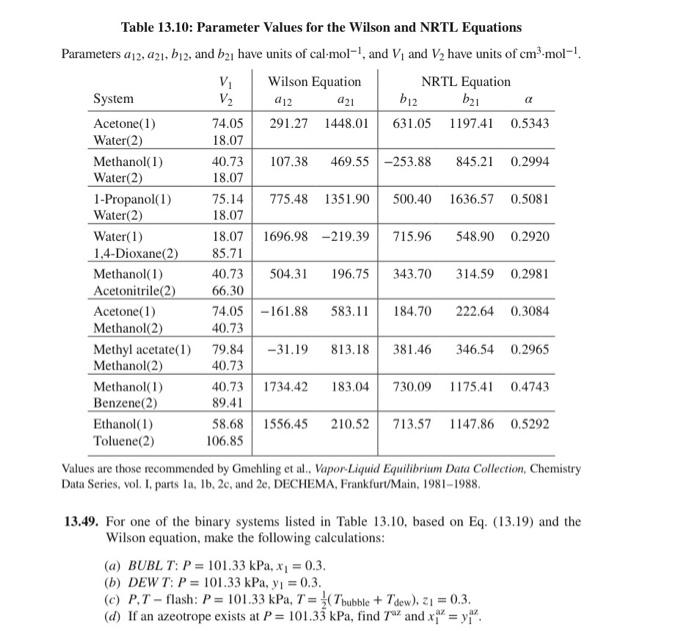
Table 13.10: Parameter Values for the Wilson and NRTL Equations Parameters a12,a21,b12, and b21 have units of cal. mol1, and V1 and V2 have units of cm3mol1. Values are those recommended by Gmehling et al.. Vapor-Liquid Equilibrium Data Collection, Chemistry Data Series, vol. I, parts la, Ib, 2c, and 2e, DECHEMA, Frankfurt/Main, 1981-1988. 13.49. For one of the binary systems listed in Table 13.10, based on Eq. (13.19) and the Wilson equation, make the following calculations: (a) BUBL T: P=101.33kPa,x1=0.3. (b) DEWT: P=101.33kPa,y1=0.3. (c) P,T - flash: P=101.33kPa,T=21(Tbubble+Tdew),z1=0.3. (d) If an azeotrope exists at P=101.33kPa, find Taz and x1az=y1az
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


