Question: Solve part (a, and c) The Levenspiel plot shown below. Three reactors are arranged in series. (1) A CSTR reactor giving Xa=0.5 (2) A PFR
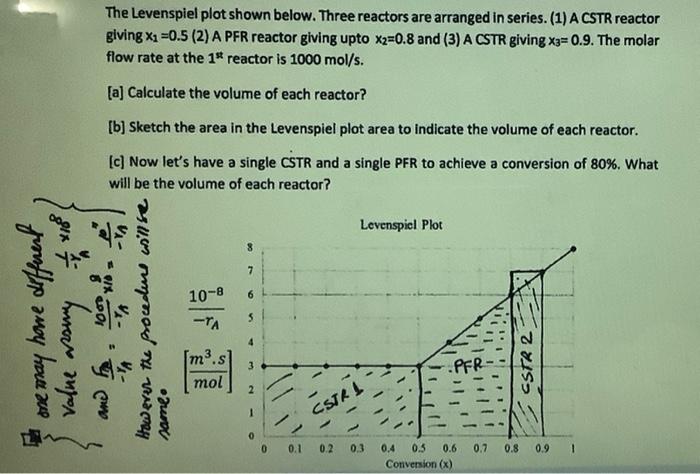
The Levenspiel plot shown below. Three reactors are arranged in series. (1) A CSTR reactor giving Xa=0.5 (2) A PFR reactor giving upto X2=0.8 and (3) A CSTR giving Xa=0.9. The molar flow rate at the 1* reactor is 1000 mol/s. [a] Calculate the volume of each reactor? [b] Sketch the area in the Levenspiel plot area to indicate the volume of each reactor. (c) Now let's have a single CSTR and a single PFR to achieve a conversion of 80%. What will be the volume of each reactor? 294% Levenspiel Plot 8 have different ** 10-8 6 value assumy to However the procedure willse -TA PFR have ane m3.s mol Y come CSTR 2 2 grund 1 0 0 0.1 0.2 0.3 0.7 0.8 0.9 0.4 0.5 0.6 Conversion (x)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


