Question: Solve part b. Hint: The answer is provided 2. A stream of hot dry air flows through a solvent recovery unit containing liquid acetone. A
 Solve part b.
Solve part b.
Hint: The answer is provided
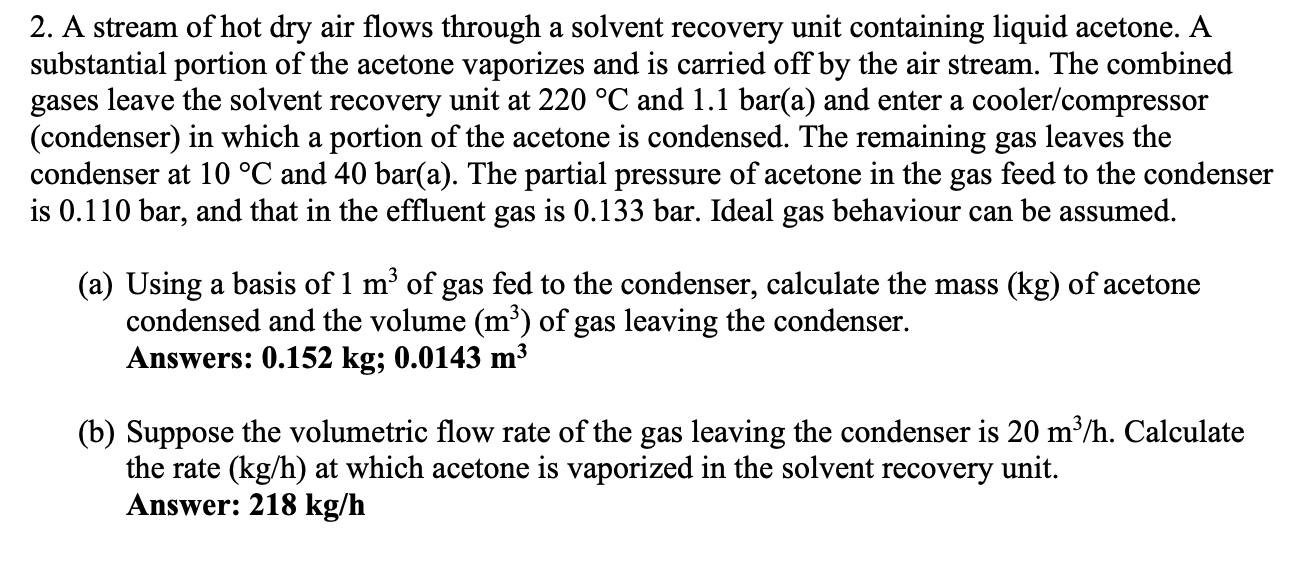
2. A stream of hot dry air flows through a solvent recovery unit containing liquid acetone. A substantial portion of the acetone vaporizes and is carried off by the air stream. The combined gases leave the solvent recovery unit at 220 C and 1.1 bar(a) and enter a cooler/compressor (condenser) in which a portion of the acetone is condensed. The remaining gas leaves the condenser at 10 C and 40 bar(a). The partial pressure of acetone in the gas feed to the condenser is 0.110 bar, and that in the effluent gas is 0.133 bar. Ideal gas behaviour can be assumed. (a) Using a basis of 1 m3 of gas fed to the condenser, calculate the mass (kg) of acetone condensed and the volume (m) of gas leaving the condenser. Answers: 0.152 kg; 0.0143 m3 (b) Suppose the volumetric flow rate of the gas leaving the condenser is 20 m3/h. Calculate the rate (kg/h) at which acetone is vaporized in the solvent recovery unit. Answer: 218 kg/h
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


