Question: solve the equations by quadratic formula showing with ICE table claculations. A,B and C solved, please solve from D to K. Cyanic acid,HCNO has a
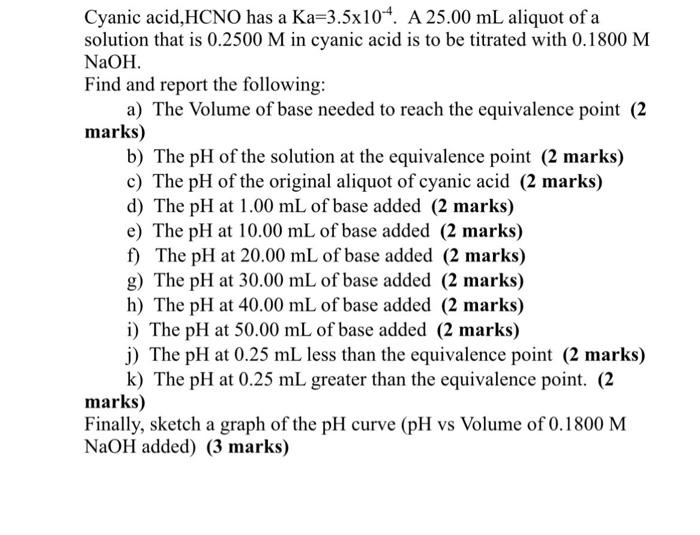
Cyanic acid,HCNO has a Ka=3.5104.A25.00mL aliquot of a solution that is 0.2500M in cyanic acid is to be titrated with 0.1800M NaOH. Find and report the following: a) The Volume of base needed to reach the equivalence point (2 marks) b) The pH of the solution at the equivalence point (2 marks) c) The pH of the original aliquot of cyanic acid (2 marks) d) The pH at 1.00mL of base added (2 marks) e) The pH at 10.00mL of base added (2 marks) f) The pH at 20.00mL of base added (2 marks) g) The pH at 30.00mL of base added (2 marks) h) The pH at 40.00mL of base added (2 marks) i) The pH at 50.00mL of base added (2 marks) j) The pH at 0.25mL less than the equivalence point (2 marks) k) The pH at 0.25mL greater than the equivalence point. (2 marks) Finally, sketch a graph of the pH curve (pH vs Volume of 0.1800M NaOH added) ( 3 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


