Question: Solve the following a . A l + H 2 S O 4 A l 2 ( S O 4 ) 3 + H 2
Solve the following
a
Which one is oxidized and which one is reduced? How much hydrogen will be produced from of
b
How much oxygen is required to produce of SO
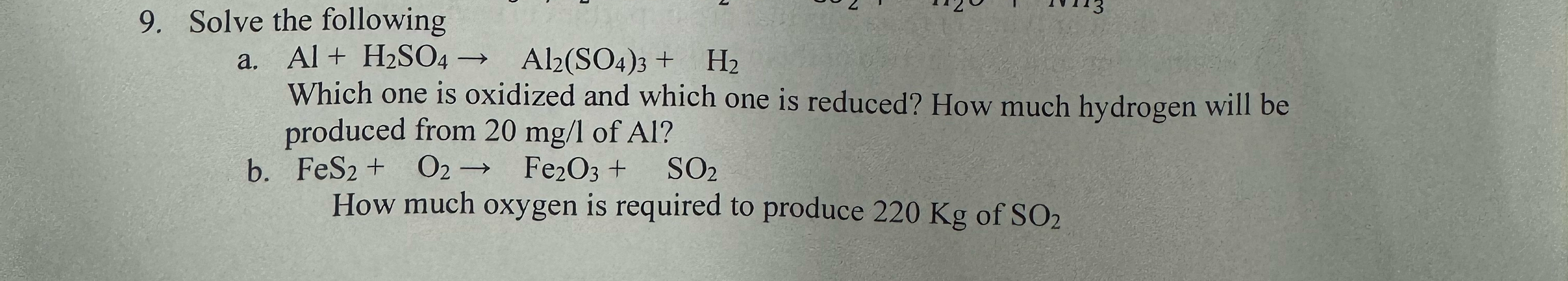
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


