Question: solve this question fast please i need so pplease answer fast in next 2 hour Direct conversion of CO2 to methanol (CH3OH) has potential to
solve this question fast please i need so pplease answer fast in next 2 hour
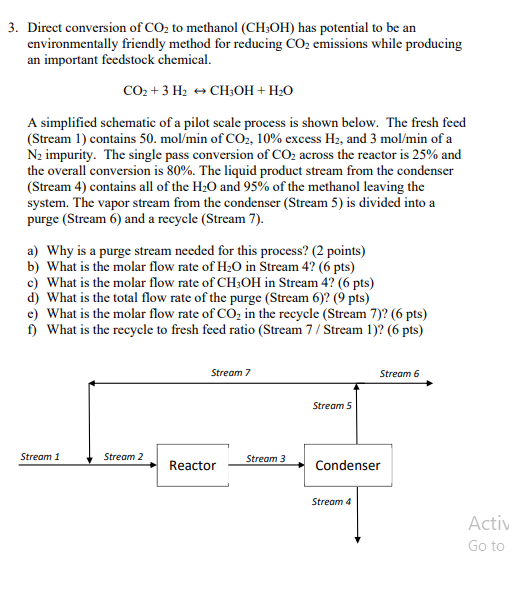
Direct conversion of CO2 to methanol (CH3OH) has potential to be an environmentally friendly method for reducing CO2 emissions while producing an important feedstock chemical. CO2+3H2CH3OH+H2O A simplified schematic of a pilot scale process is shown below. The fresh feed (Stream 1) contains 50.mol/min of CO2,10% excess H2, and 3mol/min of a N2 impurity. The single pass conversion of CO2 across the reactor is 25% and the overall conversion is 80%. The liquid product stream from the condenser (Stream 4) contains all of the H2O and 95% of the methanol leaving the system. The vapor stream from the condenser (Stream 5 ) is divided into a purge (Stream 6 ) and a recycle (Stream 7). a) Why is a purge stream needed for this process? (2 points) b) What is the molar flow rate of H2O in Stream 4? (6 pts) c) What is the molar flow rate of CH3OH in Stream 4? (6 pts) d) What is the total flow rate of the purge (Stream 6) ? ( 9pts) e) What is the molar flow rate of CO2 in the recycle (Stream 7)? (6 pts) f) What is the recycle to fresh feed ratio (Stream 7 / Stream 1)? (6 pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


