Question: Solve this question in polymath, and send the code with all details. DO NOT USE MATLAB 4. (30 pts) Consider that an irreversible, elementary reaction:
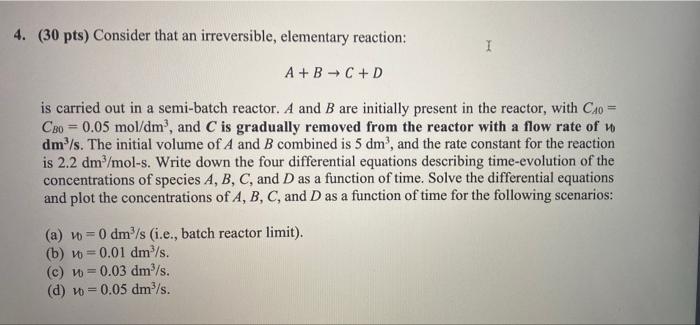
4. (30 pts) Consider that an irreversible, elementary reaction: I A + B + C +D is carried out in a semi-batch reactor. A and B are initially present in the reactor, with C_0 = CBo = 0.05 mol/dm?, and C is gradually removed from the reactor with a flow rate of dm /s. The initial volume of A and B combined is 5 dm', and the rate constant for the reaction is 2.2 dm2/mol-s. Write down the four differential equations describing time-evolution of the concentrations of species A, B, C, and D as a function of time. Solve the differential equations and plot the concentrations of A, B, C, and D as a function of time for the following scenarios: (a) v = 0 dm/s (i.e., batch reactor limit). (b) v=0.01 dm/s. (c) v = 0.03 dm/s. (d) v = 0.05 dm/s
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


