Question: Someone knowledgeable please help me with Part A, B, and C. Whoever helped before once again got it wrong. Part A: Calculate the mass of
Someone knowledgeable please help me with Part A, B, and C. Whoever helped before once again got it wrong. 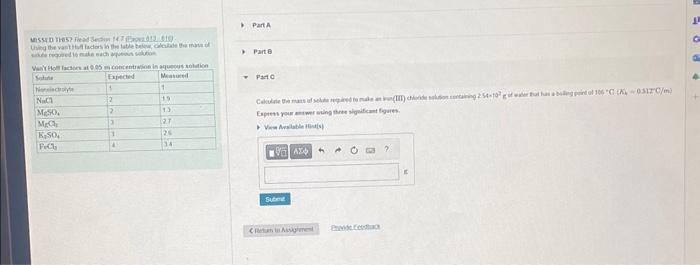
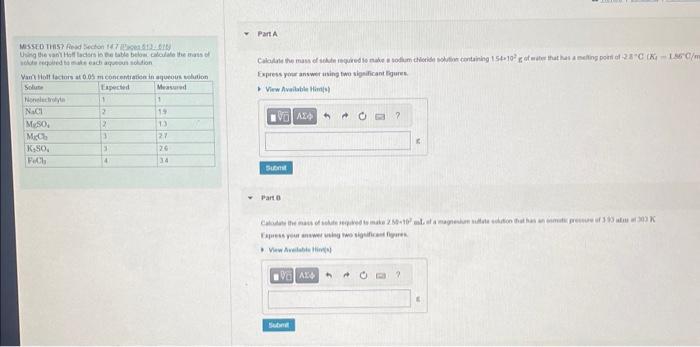
Part A: Calculate the mass of solute required to make a sodium containing 1.54x10^2g of water that has a melting point of -2.8 C (Kf=1.86 C/m)
Express your answer using two significant figures.
Part B: Calculate the mass of solute required to make 2.50x10^2 mL of magnesium sulfate solution that has an osmotic pressure of 3.93 atm at 303 K.
Express your answer using two significant figures.
Part C: Calculate the mass of solute required to make an iron (III) chloride solution containing 2.54x10^2g of water that has a boiling point of 106 C (Kb=0.512 C/m)
Express your answer using two significant figures.
2. Part 8 Pat Eapent rour antwer sing tarse siguifeat fgutes. Express your anower ating two sipeificant Egures. Part 0 Cipuess per answer whing nwo sijeificet ferrek
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


