Question: SPECIAL PROBLEM #9: Consider the scheme of elementary reactions : ki k2 A R S desired = k = 10 e -3500/T k2 = 1012
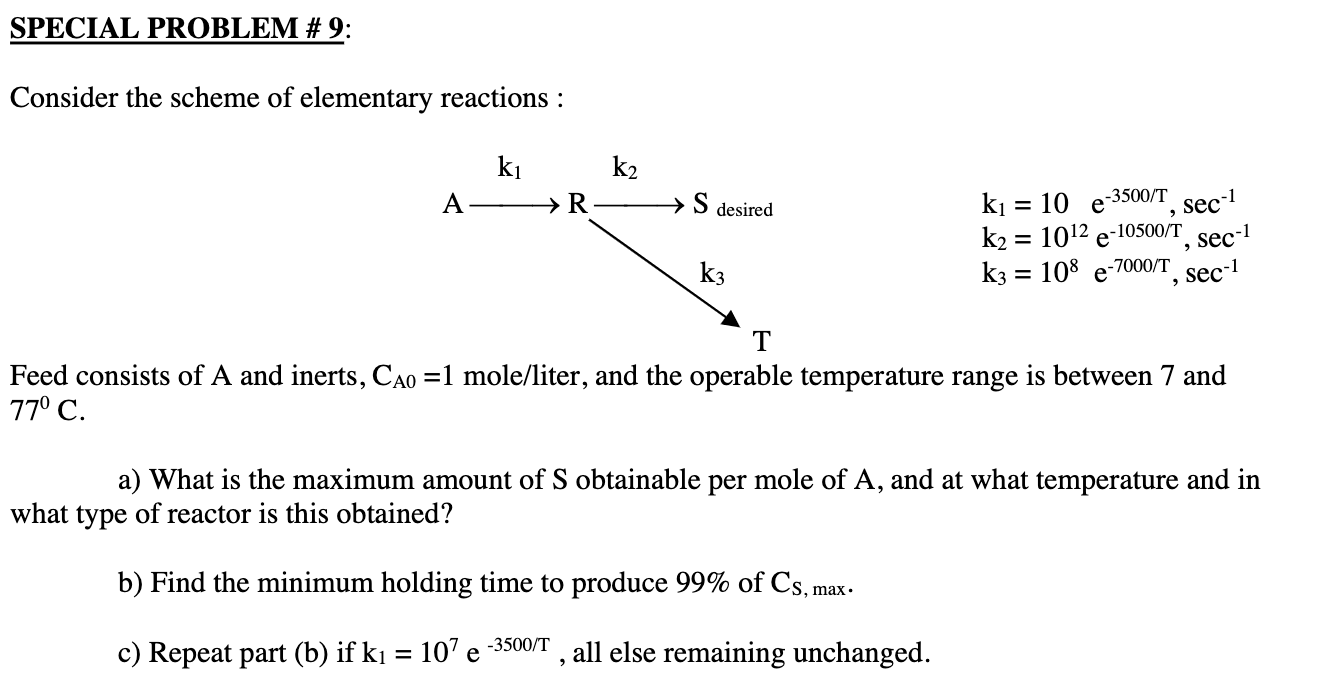
SPECIAL PROBLEM #9: Consider the scheme of elementary reactions : ki k2 A R S desired = k = 10 e -3500/T k2 = 1012 e -10500/T k3 = 108 e-7000/T, sec-1 sec-1 Sec-1 = k3 T Feed consists of A and inerts, Cao =1 mole/liter, and the operable temperature range is between 7 and 77 C. a) What is the maximum amount of S obtainable per mole of A, and at what temperature and in what type of reactor is this obtained? b) Find the minimum holding time to produce 99% of Cs, max. c) Repeat part (b) if k = 10e -3500/1 , all else remaining unchanged
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


