Question: Specify any assumptions that you make and provide justification for it . Support your answers with detailed calculations. Use MATLAB. A 1 0 0 m
Specify any assumptions that you make and provide justification for it Support your answers
with detailed calculations. Use MATLAB. A of equimolar solution of benzene and
toluene at ambient conditions and kPa is to be enriched to mole fraction of
benzene. To do so the solution is flashed at constant temperature, ie the pressure is suddenly
reduced below the bubble point pressure and thus, the solution partially evaporates; the vapors
generated are collected separately and condensed. The process is repeated until the mole
fraction of benzene reaches
Assume that the system follows Raoult's law, then what is the amount of solution with mole
fraction of benzene?
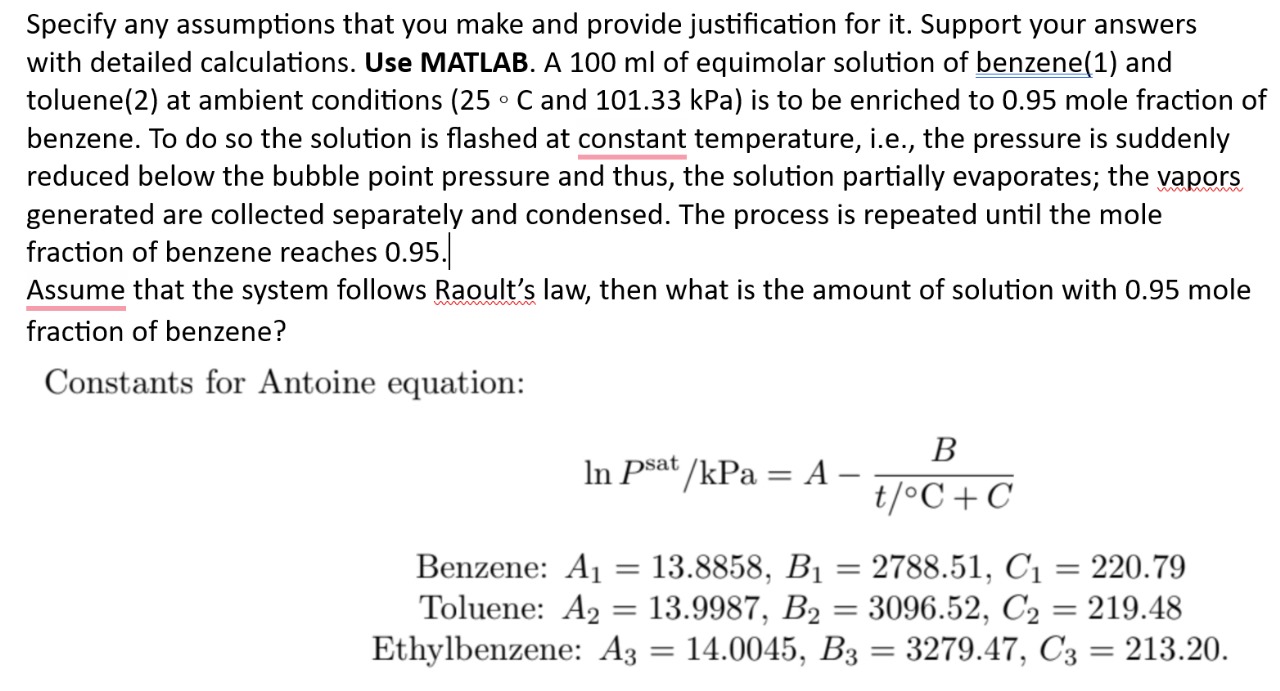
Constants for Antoine equation:
Benzene:
Toluene:
Ethylbenzene:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


