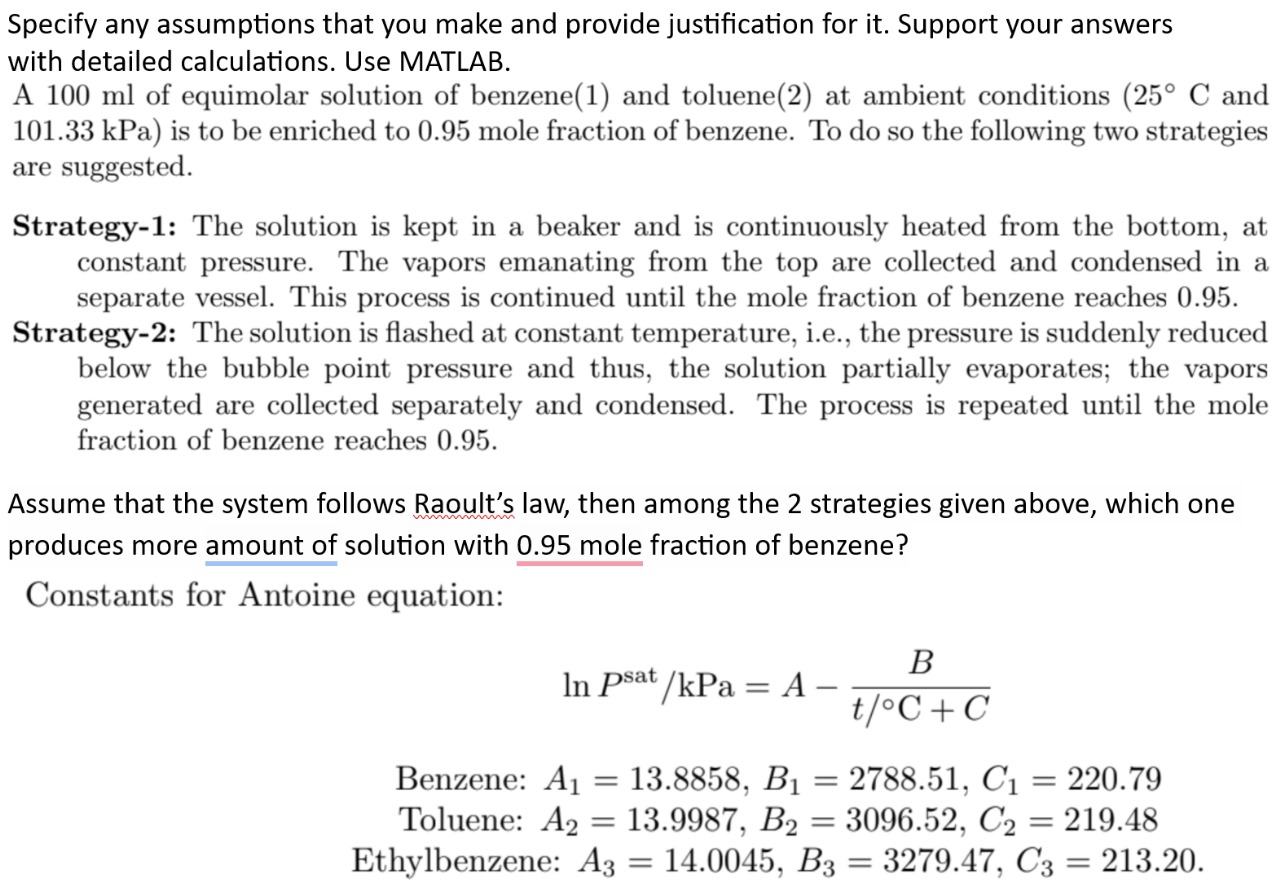
Question: Specify any assumptions that you make and provide justification for it . Support your answers with detailed calculations. Use MATLAB. A 1 0 0 m
Specify any assumptions that you make and provide justification for it Support your answers
with detailed calculations. Use MATLAB.
A of equimolar solution of benzene and toluene at ambient conditions and
kPa is to be enriched to mole fraction of benzene. To do so the following two strategies
are suggested.
Strategy: The solution is kept in a beaker and is continuously heated from the bottom, at
constant pressure. The vapors emanating from the top are collected and condensed in a
separate vessel. This process is continued until the mole fraction of benzene reaches
Strategy: The solution is flashed at constant temperature, ie the pressure is suddenly reduced
below the bubble point pressure and thus, the solution partially evaporates; the vapors
generated are collected separately and condensed. The process is repeated until the mole
fraction of benzene reaches
Assume that the system follows Raoult's law, then among the strategies given above, which one
produces more amount of solution with mole fraction of benzene?
Constants for Antoine equation:
Benzene:
Toluene:
Ethylbenzene:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


