Question: Step by step solution k 2k -> A - k R and A S k - 2 with rates TA = 3kCA - KCR-kCs, PR
Step by step solution 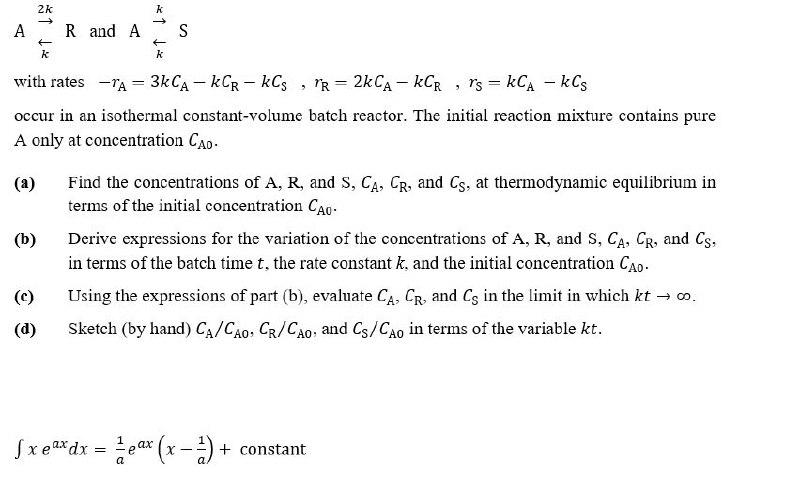
k 2k -> A - k R and A S k - 2 with rates TA = 3kCA - KCR-kCs, PR = 2kCA - KCR , rs = kCA - kCs occur in an isothermal constant-volume batch reactor. The initial reaction mixture contains pure A only at concentration CAO. (a) (b) Find the concentrations of A, R, and S, CA, CR, and Cs, at thermodynamic equilibrium in terms of the initial concentration CAO Derive expressions for the variation of the concentrations of A, R, and S, CA, CR, and Cs, in terms of the batch time t, the rate constant k, and the initial concentration CAO. Using the expressions of part (b), evaluate CA, CR, and Cs in the limit in which kt . Sketch (by hand) CA/CAO, CR/CAo, and Cs/CAo in terms of the variable kt. (d) Sxeaxdx = 2 pax (x - 2) + constant = X
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


