Question: Stoichiometry determining composition and Limiting Reactants: the percent by mass of a mixture of BaCl2. 2H20 and Na3PO4 12H20 3BaCl2 (aq) + 2Na3PO4 >
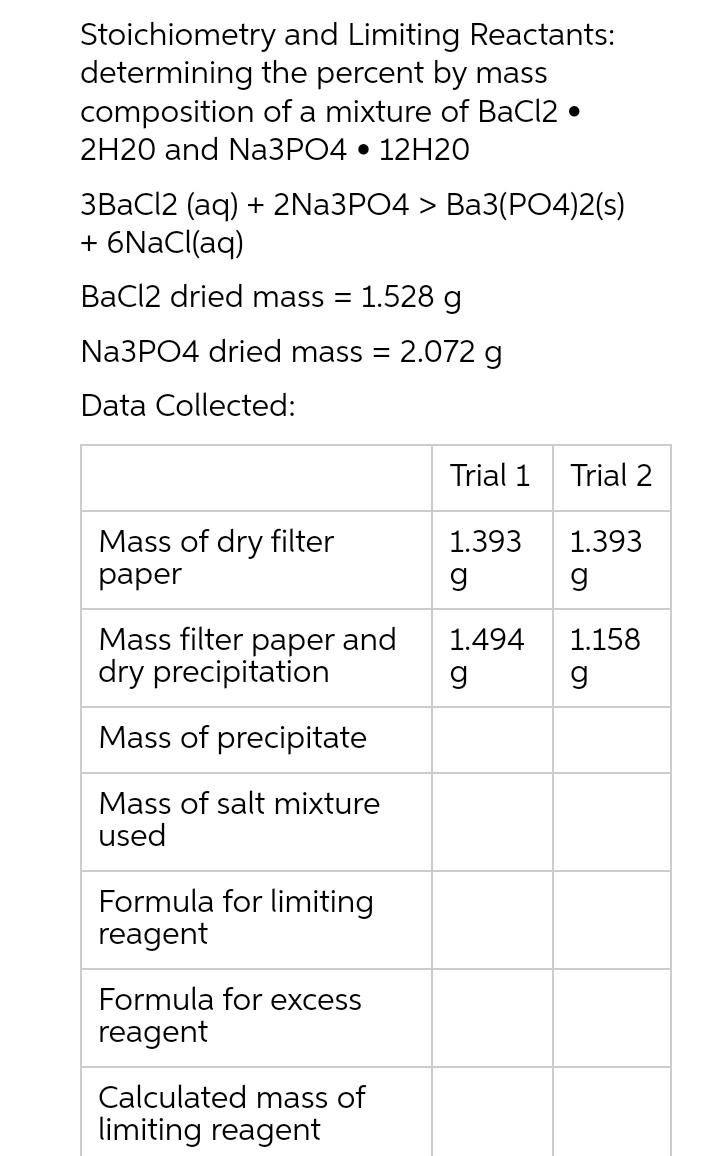
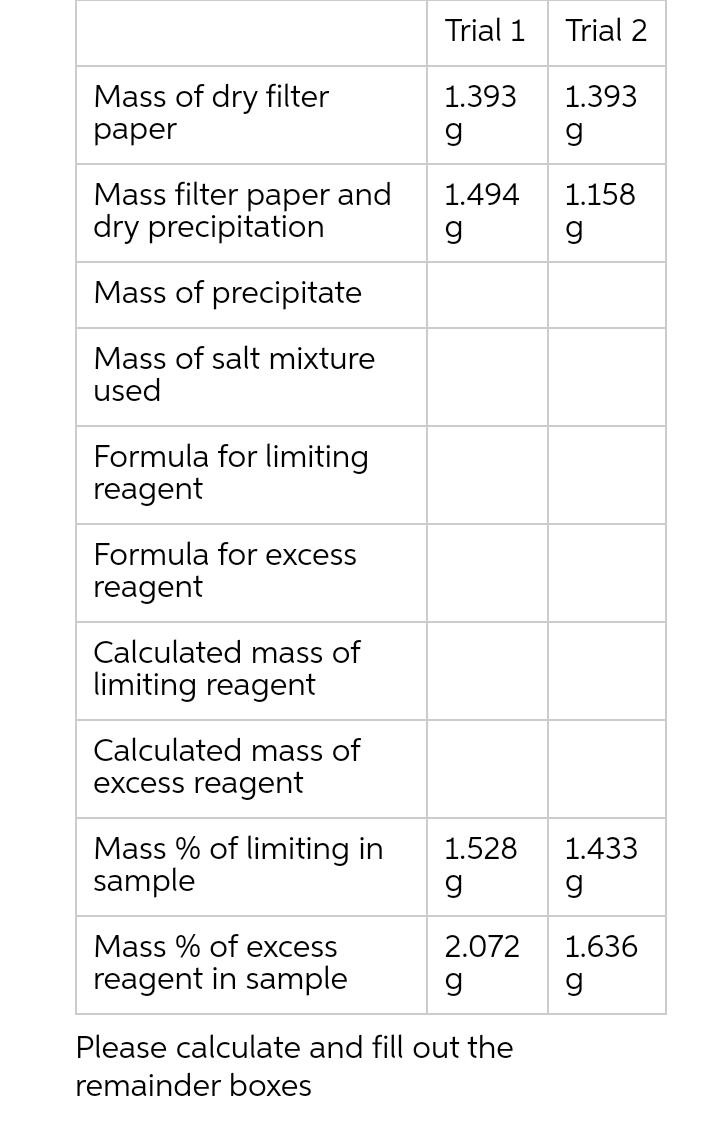
Stoichiometry determining composition and Limiting Reactants: the percent by mass of a mixture of BaCl2. 2H20 and Na3PO4 12H20 3BaCl2 (aq) + 2Na3PO4 > Ba3(PO4)2(s) + 6NaCl(aq) BaCl2 dried mass = 1.528 g Na3PO4 dried mass = 2.072 g Data Collected: Mass of dry filter paper Mass filter paper and dry precipitation Mass of precipitate Mass of salt mixture used Formula for limiting reagent Formula for excess reagent Calculated mass of limiting reagent Trial 1 1.393 g 1.494 g Trial 2 1.393 g 1.158 g Mass of dry filter paper Mass filter paper and dry precipitation Mass of precipitate Mass of salt mixture used Formula for limiting reagent Formula for excess reagent Calculated mass of limiting reagent Calculated mass of excess reagent Mass % of limiting in sample Mass % of excess reagent in sample Trial 1 1.393 g 1.494 g 1.528 g 2.072 g Please calculate and fill out the remainder boxes Trial 2 1.393 g 1.158 g 1.433 g 1.636 g
Step by Step Solution
3.61 Rating (158 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


