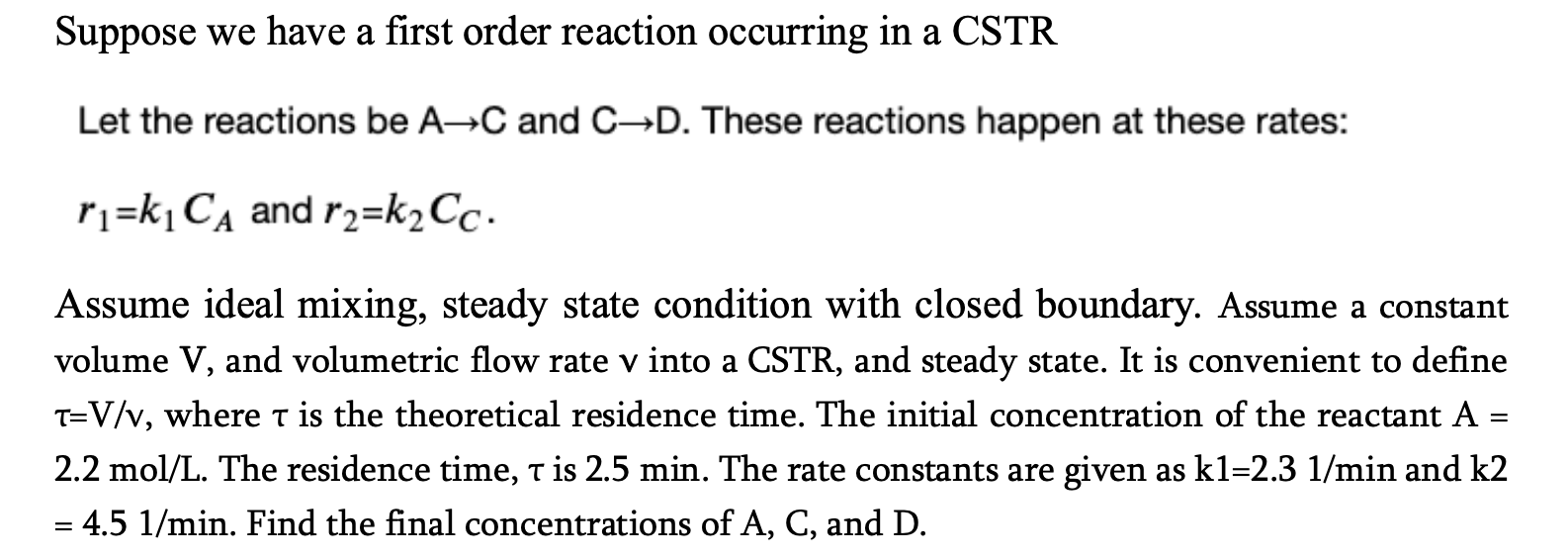
Question: Suppose we have a first order reaction occurring in a CSTR Let the reactions be A C and C D . These reactions happen at
Suppose we have a first order reaction occurring in a CSTR
Let the reactions be and These reactions happen at these rates:
and
Assume ideal mixing, steady state condition with closed boundary. Assume a constant
volume and volumetric flow rate into a CSTR and steady state. It is convenient to define
where is the theoretical residence time. The initial concentration of the reactant
The residence time, is min. The rate constants are given as and
Find the final concentrations of and
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


