Question: Suppose we have a silicate glass composition. This composition, in percent by weight, is as follows: 15Na 2 O + 5K 2 O + 2.5MgO
Suppose we have a silicate glass composition. This composition, in percent by weight, is as follows:
15Na2O + 5K2O + 2.5MgO + 9.5CaO + 6 BaO + 5Al2O3 + 57 SiO2.
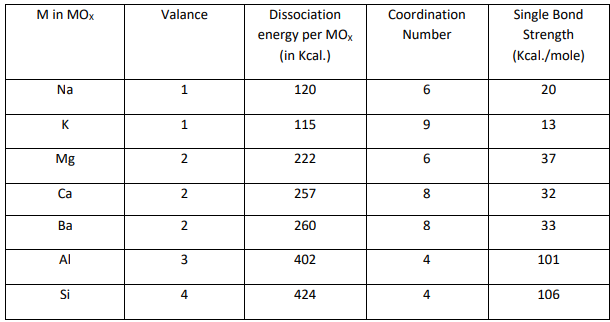
First, convert this composition to mole percent. Classify each glass oxide according to Sun's Bond Strength criteria. Now, if we take this classification process as an example and apply the same process to this glass composition, how would you evaluate the glass? Does glass or not?
Oxides (Molecular Weight): Na2O:(61.97), K2O:(94.18), MgO:(40.31), CaO:(56.08), BaO:(153.33), Al2O3 (101.96), SiO2:(60.08)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


