Question: Suppose you need to differentiate the solids in each pair using their solubility difference in water and ethanol (as shown in the table). So you
Suppose you need to differentiate the solids in each pair using their solubility difference in water and ethanol (as shown in the table). So you dissolve 0.40 g of each solid into 10 mL each solvent (Each solution composes one solvent and one solute). The solids in which pair CANNOT be differentiated using their solubility difference in water and ethanol?
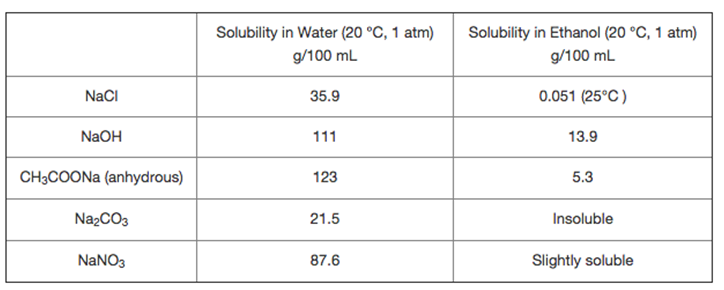
- Na 2 CO 3 and CH 3 COONa (anhydrous)
- NaOH and NaOH 3
- NaCI and NaOH
- NaOH and CH 3 COONa (anhydrous)
- NaOH 3 and CH 3 COONa (anhydrous)
NaCl NaOH CH3COONa (anhydrous) NaCO3 NaNO3 Solubility in Water (20 C, 1 atm) g/100 mL 35.9 111 123 21.5 87.6 Solubility in Ethanol (20 C, 1 atm) g/100 mL 0.051 (25C) 13.9 5.3 Insoluble Slightly soluble
Step by Step Solution
3.42 Rating (161 Votes )
There are 3 Steps involved in it
To determine which pair of solids cannot be differentiated using their solubility differences in wat... View full answer

Get step-by-step solutions from verified subject matter experts


