Question: Switch Sn Voltmeter Salt Bridge Wire Metal X 1.0 M Sn(NO) 1.0 M X(NO), An electrochemical cell is constructed with an open switch, as
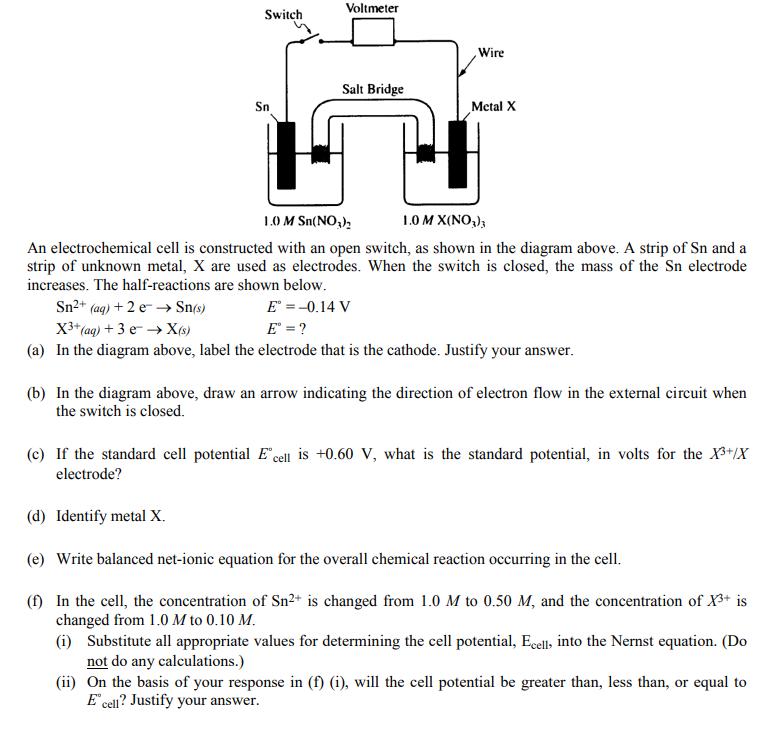
Switch Sn Voltmeter Salt Bridge Wire Metal X 1.0 M Sn(NO) 1.0 M X(NO), An electrochemical cell is constructed with an open switch, as shown in the diagram above. A strip of Sn and a strip of unknown metal, X are used as electrodes. When the switch is closed, the mass of the Sn electrode increases. The half-reactions are shown below. Sn+ (aq) + 2e Sn(s) E = -0.14 V E = ? X+ (aq) + 3 e X(s) (a) In the diagram above, label the electrode that is the cathode. Justify your answer. (b) In the diagram above, draw an arrow indicating the direction of electron flow in the external circuit when the switch is closed. (c) If the standard cell potential E cell is +0.60 V, what is the standard potential, in volts for the X+/X electrode? (d) Identify metal X. (e) Write balanced net-ionic equation for the overall chemical reaction occurring in the cell. (f) In the cell, the concentration of Sn+ is changed from 1.0 M to 0.50 M, and the concentration of X+ is changed from 1.0 M to 0.10 M. (i) Substitute all appropriate values for determining the cell potential, Ecell, into the Nernst equation. (Do not do any calculations.) (ii) On the basis of your response in (f) (i), will the cell potential be greater than, less than, or equal to E cell? Justify your answer.
Step by Step Solution
3.45 Rating (155 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


