Question: T LAUNCH TUTORIAL LESSON Seep 12 Question (4 points) COAST Tutorial Problem The following reaction has these standard thermodynamic parameters: AH - - 36,1 kJ/mol


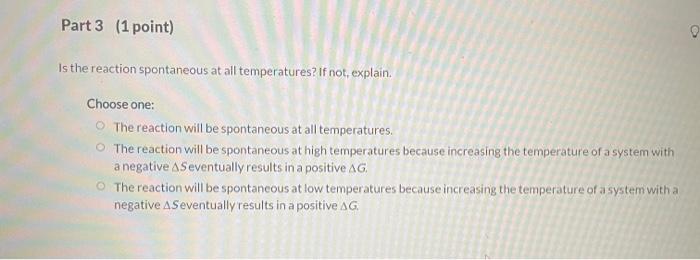

T LAUNCH TUTORIAL LESSON Seep 12 Question (4 points) COAST Tutorial Problem The following reaction has these standard thermodynamic parameters: AH - - 36,1 kJ/mol and AS x = -67.3 Jmol. K). Cle)+($) 18) 1st attempt Part 1 (1 point) Is the reaction exothermic or endothermic? Choose one: O endothermic exothermic Part 2 (1 point) Does the negative entropy change make sense? Why or why not? Choose one: o Yes, because the number of independently moving particles decreases. o Yes, because the number of independently moving particles increases No, because the number of independently moving particles decreases. No, because the number of independently moving particles increases. Part 3 (1 point) Is the reaction spontaneous at all temperatures? If not, explain. Choose one: The reaction will be spontaneous at all temperatures. The reaction will be spontaneous at high temperatures because increasing the temperature of a system with a negative & Seventually results in a positive AG The reaction will be spontaneous at low temperatures because increasing the temperature of a system with a negative AS eventually results in a positive AG Part 4 (1 point) Calculate the temperature at which the reaction becomes nonspontaneous K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


