Question: Table 1 Consider the following relation: k=koexp(-E/(RT)) that relates k (reaction rate constant) to temperature (T). R is the ideal gas constant (known) while ko
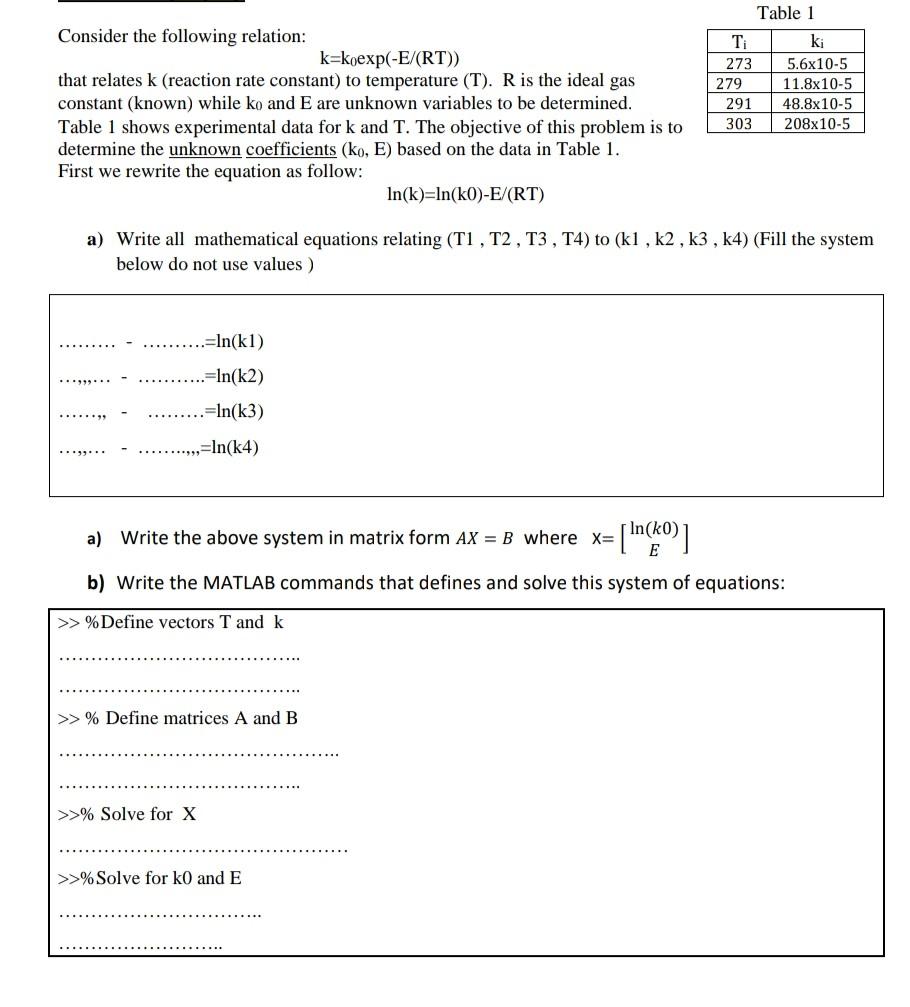
Table 1 Consider the following relation: k=koexp(-E/(RT)) that relates k (reaction rate constant) to temperature (T). R is the ideal gas constant (known) while ko and E are unknown variables to be determined. Table 1 shows experimental data for k and T. The objective of this problem is to determine the unknown coefficients (ko, E) based on the data in Table 1. First we rewrite the equation as follow: In(k)=In(ko)-E/(RT) T 273 279 291 303 ki 5.6x10-5 11.8x10-5 48.8x10-5 208x10-5 a) Write all mathematical equations relating (T1, T2, T3, T4) to (kl , k2, k3 , k4) (Fill the system below do not use values ) =In(kl) .=ln(k2) =In(3) =ln(k4) a) Write the above system in matrix form AX = B where x= [ln(ko)] E b) Write the MATLAB commands that defines and solve this system of equations: >>% Define vectors T and k >>% Define matrices A and B >>% Solve for X >>% Solve for k0 and E
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


