Question: Table 1: Equilibrium data Temperature K Mole fraction C 1 409.3 136.1 0 0 402.6 129.4 0.00 0.230 3926 119.4 0.250 0.514 383.8 1106 0.485
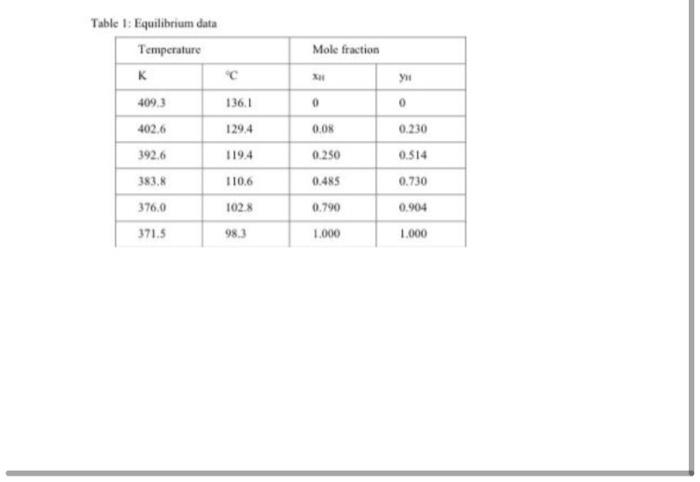

Table 1: Equilibrium data Temperature K Mole fraction C 1 409.3 136.1 0 0 402.6 129.4 0.00 0.230 3926 119.4 0.250 0.514 383.8 1106 0.485 0.730 376.0 1028 0.790 0.904 371.5 98.3 1.000 1.000 1. Rectification of a Heptane- Ethyl Benzene Mixture. A saturated liquid feed of 200 mollh at A the boiling point containing 42 mol% heptane and 58 mol% ethyl benzene is to be fractionated at 101.32kpe abs to a distillate containing 97 mol % heptane and a bottoms containing I'moli, heptane. The reflux ratio used is 2.5: 1. Caleulate the distillate, bottoms, theoretical number of trays and actual number of trays, and also the feed tray, number. The percent efficiency is 77%. Equilibrium data (Table) is given below at 101,32kPa abs pressure for mole fraction n-heptane n and XH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


