Question: Table 6. Steel Bolt Specific Heat Data Bolt T, (C) Water T (C) Water and Bolt T, (C) Water AT = T- T Bolt
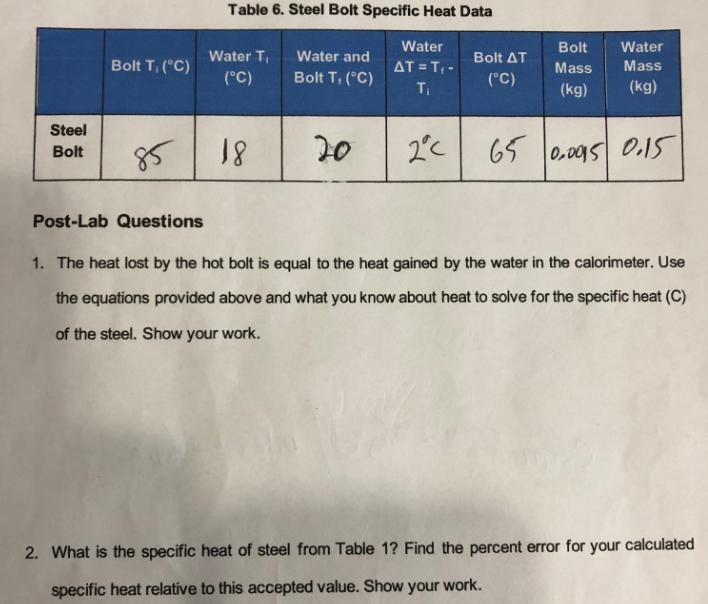
Table 6. Steel Bolt Specific Heat Data Bolt T, (C) Water T (C) Water and Bolt T, (C) Water AT = T- T Bolt Bolt AT (C) Water Mass Mass (kg) (kg) Steel Bolt 85 18 20 2C 65 0.0015 0.15 Post-Lab Questions 1. The heat lost by the hot bolt is equal to the heat gained by the water in the calorimeter. Use the equations provided above and what you know about heat to solve for the specific heat (C) of the steel. Show your work. 2. What is the specific heat of steel from Table 1? Find the percent error for your calculated specific heat relative to this accepted value. Show your work.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


