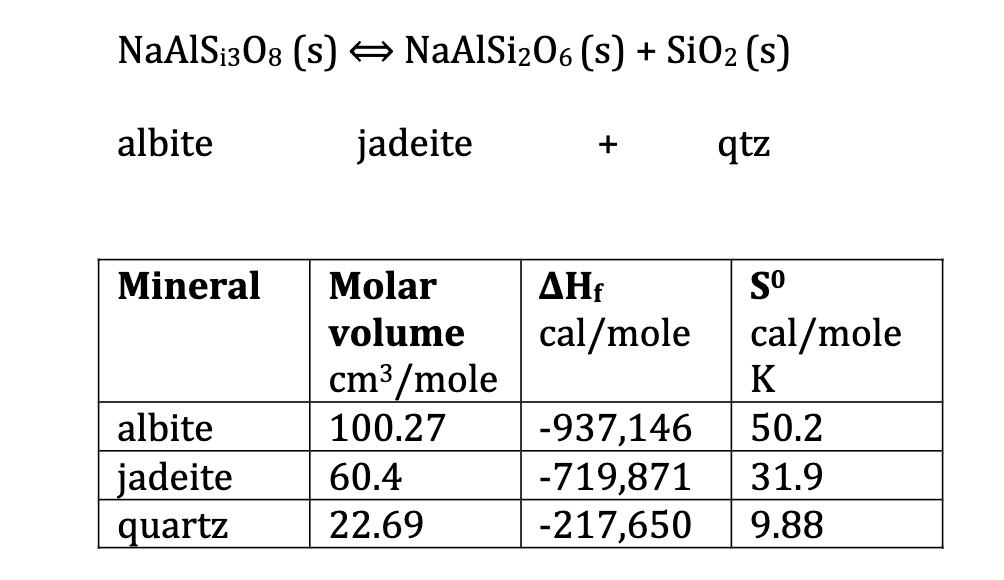
Question: table [ [ N a A l S i 3 O 8 ( s ) > N a A l S i 2 O
table
What would be favored phase assemblage at the following PT cobditons:
kb K kbar, K kbar, K
calculate the enthalpy H and entropy S of albite at K using the following standard state values and polynomial for isobaric heat capacity:
S at K is Jmole K
Delta fH kJmol
Cp TxTxT
Do it the hard way by assuming that heat capacity does increase with temperature.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


