Question: Taking again the reaction Cr3+ + 3Ce4+ + 4 H 0 3 Cro- + 3Ce3+ +8H+ with the rate law d[Cr3+) [Ce4+]2[Cr3+) = K -
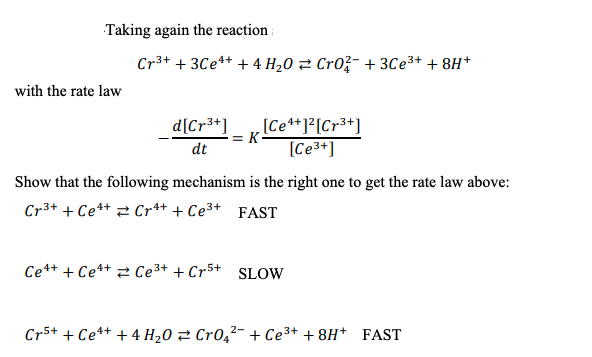
Taking again the reaction Cr3+ + 3Ce4+ + 4 H 0 3 Cro- + 3Ce3+ +8H+ with the rate law d[Cr3+) [Ce4+]2[Cr3+) = K - dt [Ce3+] Show that the following mechanism is the right one to get the rate law above: Cr3+ + Ce4+ Cr4+ + Ce3+ FAST Ce4+ + Ce++ Ce3+ + Cr5+ SLOW Cr5+ + Ce4+ + 4 H20 = Cro_2- + Ce3+ +8H+ FAST
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


