Question: Task 3: Perfect gases Two perfect gases A and B are inside a closed cylinder and are separated from each other by a frictionless
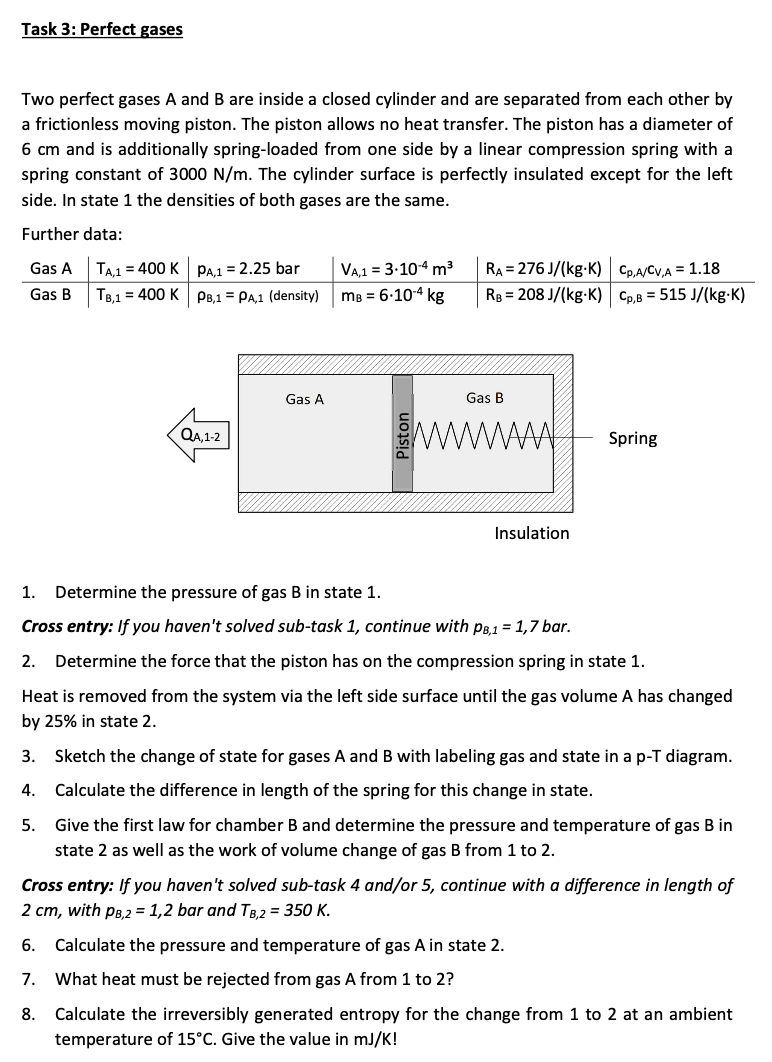
Task 3: Perfect gases Two perfect gases A and B are inside a closed cylinder and are separated from each other by a frictionless moving piston. The piston allows no heat transfer. The piston has a diameter of 6 cm and is additionally spring-loaded from one side by a linear compression spring with a spring constant of 3000 N/m. The cylinder surface is perfectly insulated except for the left side. In state 1 the densities of both gases are the same. Further data: Gas A Gas B TA,1 = 400 K TB,1 = 400 K 5. PA,1 = 2.25 bar PB,1 PA,1 (density) QA, 1-2 Gas A VA,1 = 3.10-4 m MB = 6.10-4 kg RA= 276 J/(kg-K) Cp,A/CV,A = 1.18 RB = 208 J/(kg-K) Cp,B=515 J/(kg.K) Gas B Insulation Spring 1. Determine the pressure of gas B in state 1. Cross entry: If you haven't solved sub-task 1, continue with PB,1 = 1,7 bar. 2. Determine the force that the piston has on the compression spring in state 1. Heat is removed from the system via the left side surface until the gas volume A has changed by 25% in state 2. 3. Sketch the change of state for gases A and B with labeling gas and state in a p-T diagram. Calculate the difference in length of the spring for this change in state. 4. Give the first law for chamber B and determine the pressure and temperature of gas B in state 2 as well as the work of volume change of gas B from 1 to 2. Cross entry: If you haven't solved sub-task 4 and/or 5, continue with a difference in length of 2 cm, with PB,2 = 1,2 bar and TB,2 = 350 K. 6. Calculate the pressure and temperature of gas A in state 2. 7. What heat must be rejected from gas A from 1 to 2? 8. Calculate the irreversibly generated entropy for the change from 1 to 2 at an ambient temperature of 15C. Give the value in mJ/K!
Step by Step Solution
3.38 Rating (167 Votes )
There are 3 Steps involved in it
Diagram ... View full answer

Get step-by-step solutions from verified subject matter experts


