Question: Two perfect gases A and B are inside a closed cylinder and are separated from each other by a frictionless moving piston. The piston allows
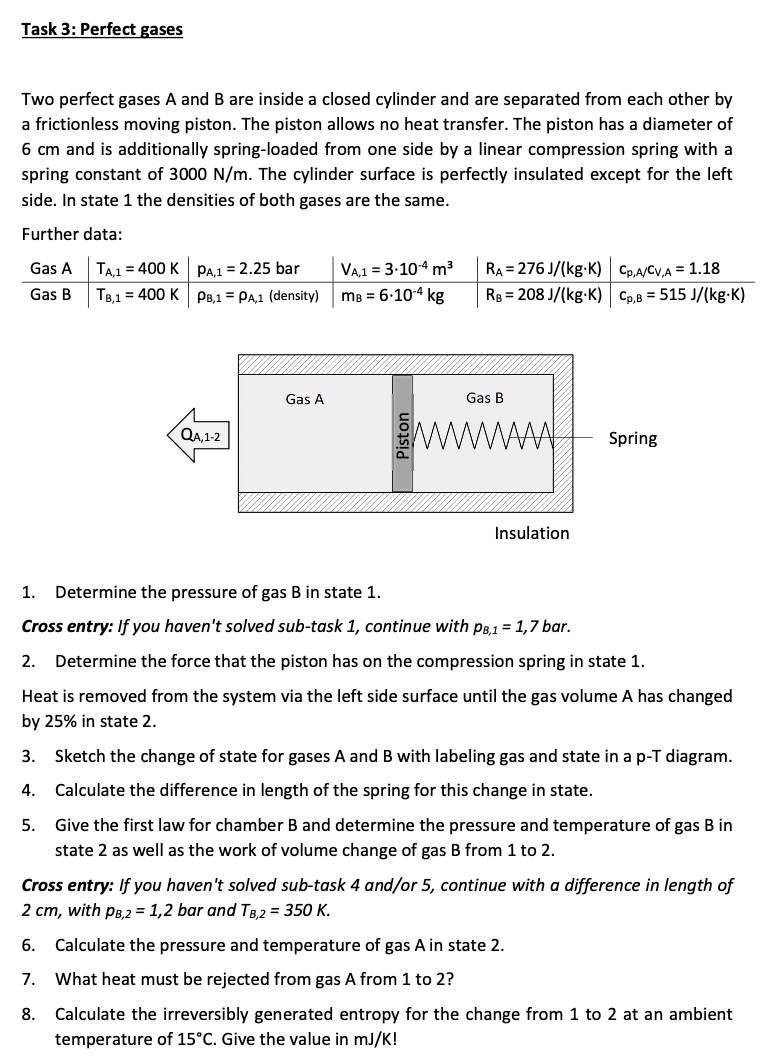
Two perfect gases A and B are inside a closed cylinder and are separated from each other by
a frictionless moving piston. The piston allows no heat transfer. The piston has a diameter of
and is additionally springloaded from one side by a linear compression spring with a
spring constant of The cylinder surface is perfectly insulated except for the left
side. In state the densities of both gases are the same.
Further data:
ng
Determine the pressure of gas in state
Cross entry: If you haven't solved subtask continue with bar.
Determine the force that the piston has on the compression spring in state
Heat is removed from the system via the left side surface until the gas volume A has changed
by in state
Sketch the change of state for gases A and B with labeling gas and state in a pT diagram.
Calculate the difference in length of the spring for this change in state.
Give the first law for chamber B and determine the pressure and temperature of gas B in
state as well as the work of volume change of gas B from to
Cross entry: If you haven't solved subtask andor continue with a difference in length of
with bar and
Calculate the pressure and temperature of gas in state
What heat must be rejected from gas A from to
Calculate the irreversibly generated entropy for the change from to at an ambient
temperature of Give the value in
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


