Question: Temperature (C) Vapor pressure for four liquids as a function of temperature. What is the vapor pressure of ethylene glycol at its normal boiling point?
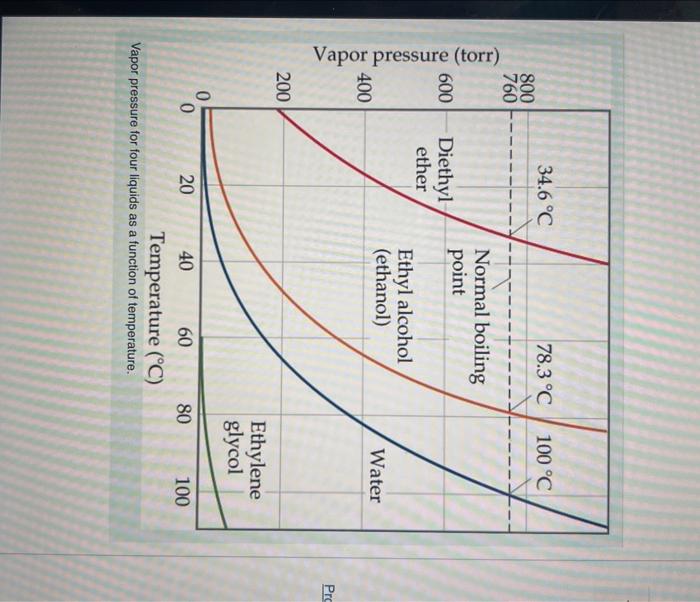
Temperature (C) Vapor pressure for four liquids as a function of temperature. What is the vapor pressure of ethylene glycol at its normal boiling point? Express your answer with the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


