Question: temperatures and pressures being equal. 5. Use F(v)dv function for v to find v3 for ideal gas molecule. Does v3 equal to v2v
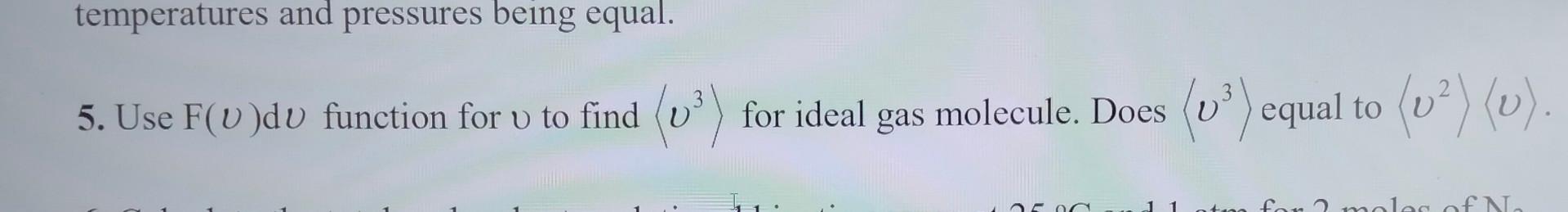
temperatures and pressures being equal. 5. Use F(v)dv function for v to find v3 for ideal gas molecule. Does v3 equal to v2v
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


