Question: Use the References to access important values if needed for this question The freezing point of water is 0.00C at 1 atmosphere. A student dissolves
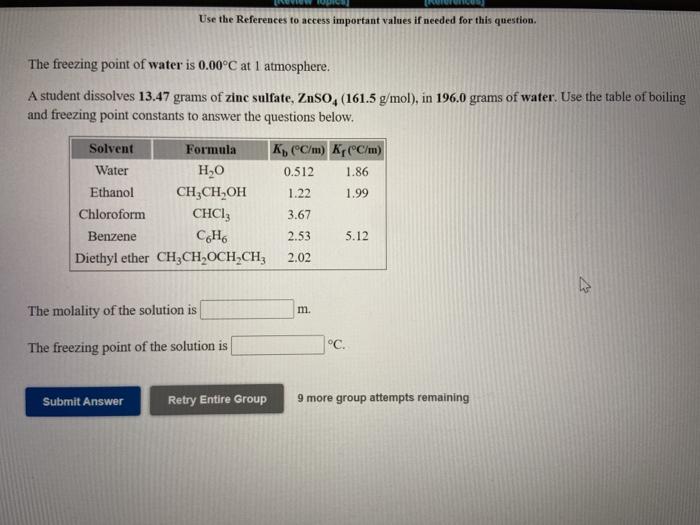
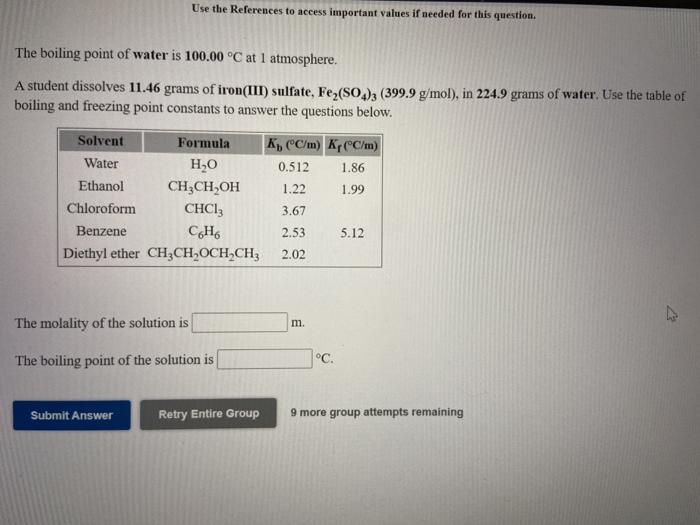
Use the References to access important values if needed for this question The freezing point of water is 0.00C at 1 atmosphere. A student dissolves 13.47 grams of zinc sulfate, ZnSO (161.5 g/mol), in 196.0 grams of water. Use the table of boiling and freezing point constants to answer the questions below. Solvent Formula K C/m) Kr(C/m) Water HO 0.512 1.86 Ethanol CH3CH,OH 1.22 1.99 Chloroform CHCI 3.67 Benzene CH 2.53 5.12 Diethyl ether CH2CH OCH-CH2 2.02 The molality of the solution is m. The freezing point of the solution is C Submit Answer Retry Entire Group 9 more group attempts remaining Use the References to access important values if needed for this question. The boiling point of water is 100.00 C at 1 atmosphere. A student dissolves 11.46 grams of iron(III) sulfate, Fe2(SO2), (399.9 g/mol), in 224.9 grams of water. Use the table of boiling and freezing point constants to answer the questions below. Solvent Formula K(C/m) KCC/m) Water HO 0.512 1.86 Ethanol 3, 1.22 1.99 Chloroform CHCI: 3.67 Benzene CH 2.53 5.12 Diethyl ether CH2CH OCH CH3 2.02 The molality of the solution is m. The boiling point of the solution is C. Submit Answer Retry Entire Group 9 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


