Question: The answer is -1638 kJ. I do not understand how I can get -1638 kJ as my final answer. Can you please help me understand
The answer is -1638 kJ. I do not understand how I can get -1638 kJ as my final answer. Can you please help me understand how I get to the final answer? Thank you. 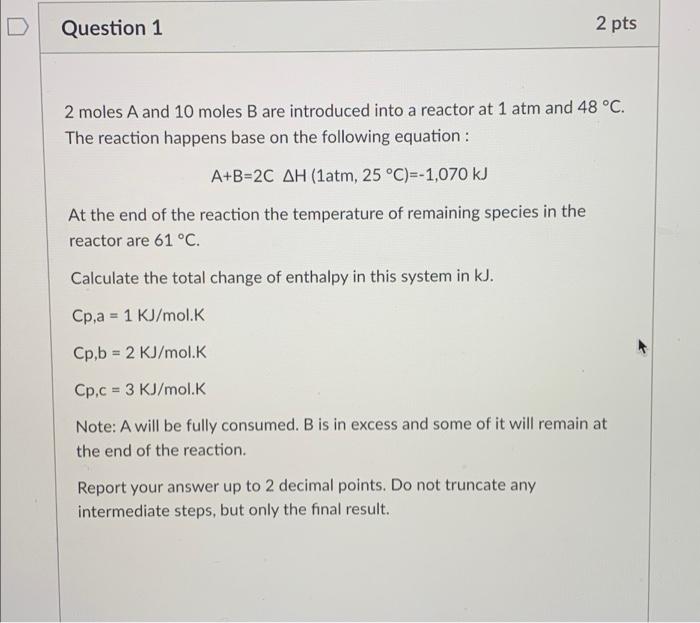

D Question 1 2 pts 2 moles A and 10 moles B are introduced into a reactor at 1 atm and 48 C. The reaction happens base on the following equation: A+B=2C AH (1atm, 25 C)=-1,070 kJ At the end of the reaction the temperature of remaining species in the reactor are 61 C. Calculate the total change of enthalpy in this system in kJ. Cp.a = 1 kJ/mol.K Cp,b = 2 KJ/mol.K Cp,c = 3 KJ/mol.K Note: A will be fully consumed. B is in excess and some of it will remain at the end of the reaction. Report your answer up to 2 decimal points. Do not truncate any intermediate steps, but only the final result
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


