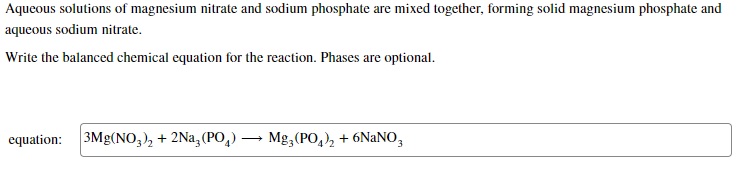
Question: The answer says it's wrong but I'm not sure why Aqueous solutions of magnesium nitrate and sodium phosphate are mixed together, forming solid magnesium phosphate
The answer says it's wrong but I'm not sure why
Aqueous solutions of magnesium nitrate and sodium phosphate are mixed together, forming solid magnesium phosphate and aqueous sodium nitrate. Write the balanced chemical equation for the reaction. Phases are optional. \begin{tabular}{l|l} equation: 3Mg(NO3)2+2Na3(PO4)Mg3(PO4)2+6NaNO3 \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


