Question: The citrate is tricky because it is triprotic - use HC6H5O72 as your acid in the calculation, but since citric acid is triprotic you only
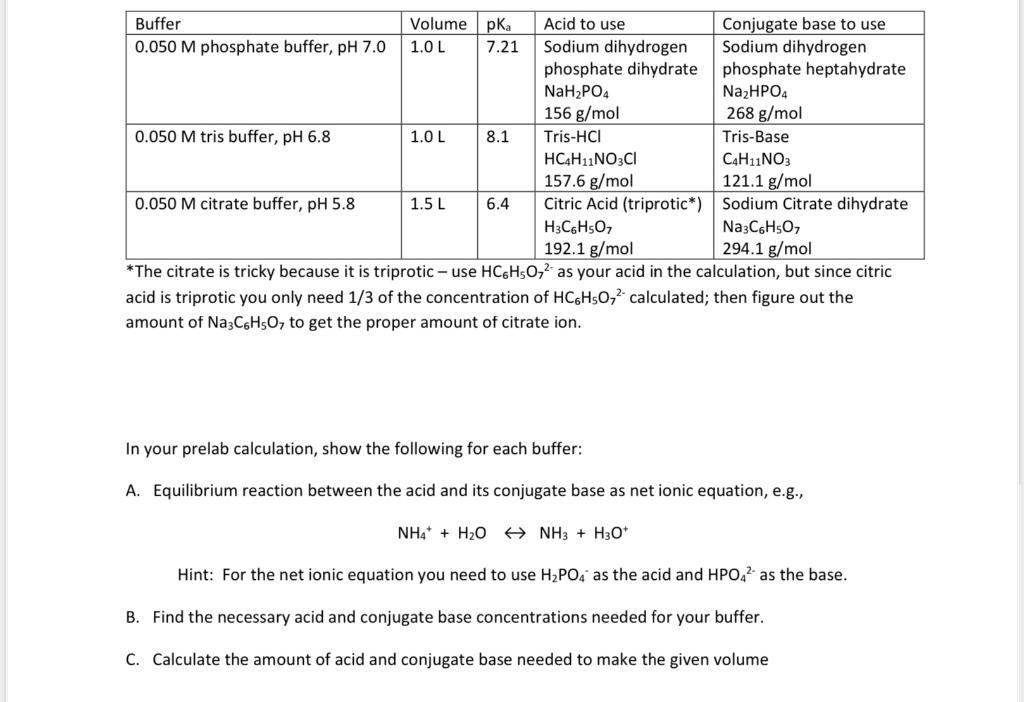
The citrate is tricky because it is triprotic - use HC6H5O72 as your acid in the calculation, but since citric acid is triprotic you only need 1/3 of the concentration of HC6H5O72 calculated; then figure out the amount of Na3C6H5O7 to get the proper amount of citrate ion. In your prelab calculation, show the following for each buffer: A. Equilibrium reaction between the acid and its conjugate base as net ionic equation, e.g., NH4++H2ONH3+H3O+ Hint: For the net ionic equation you need to use H2PO4as the acid and HPO42 as the base. B. Find the necessary acid and conjugate base concentrations needed for your buffer. C. Calculate the amount of acid and conjugate base needed to make the given volume
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


