Question: The data in the table concern the lactonization of hydroxyvaleric acid at 25 degrees Celsius. They give the concentration C(t) of this acid in moles
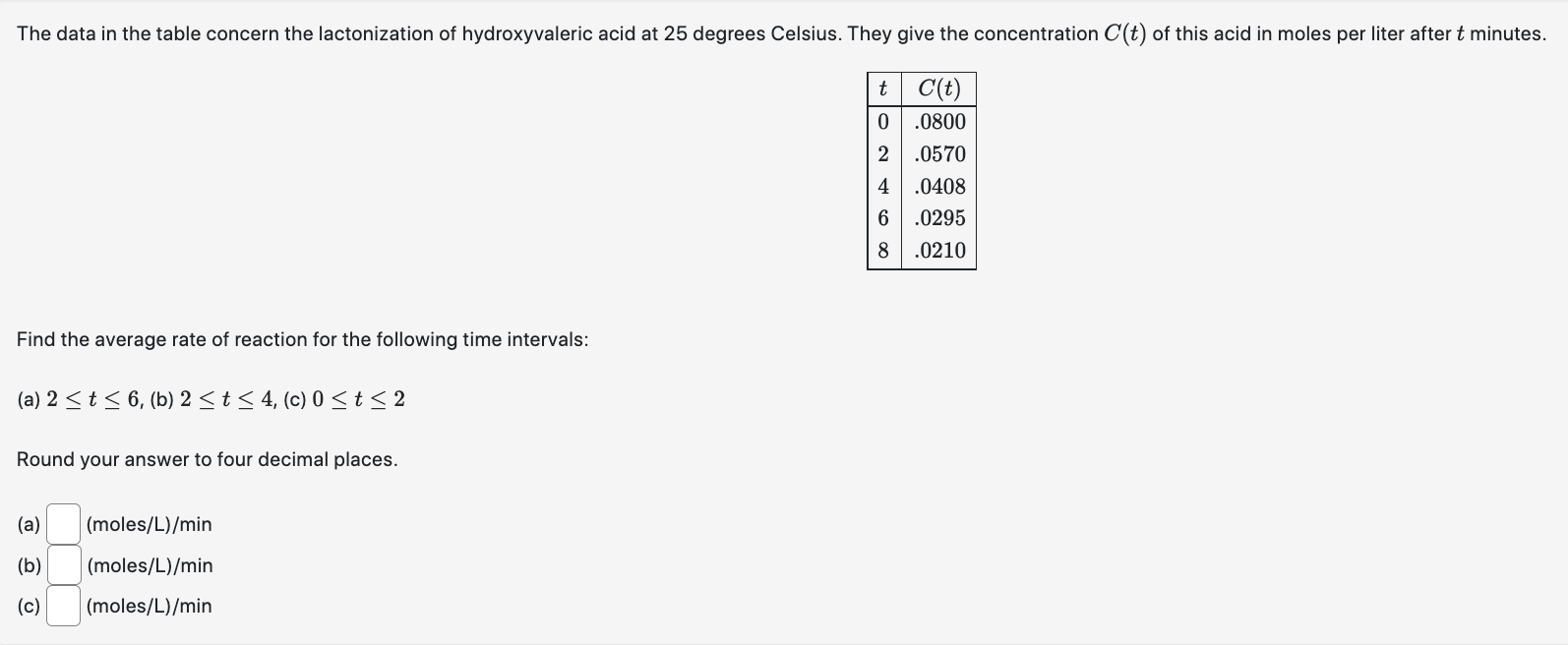



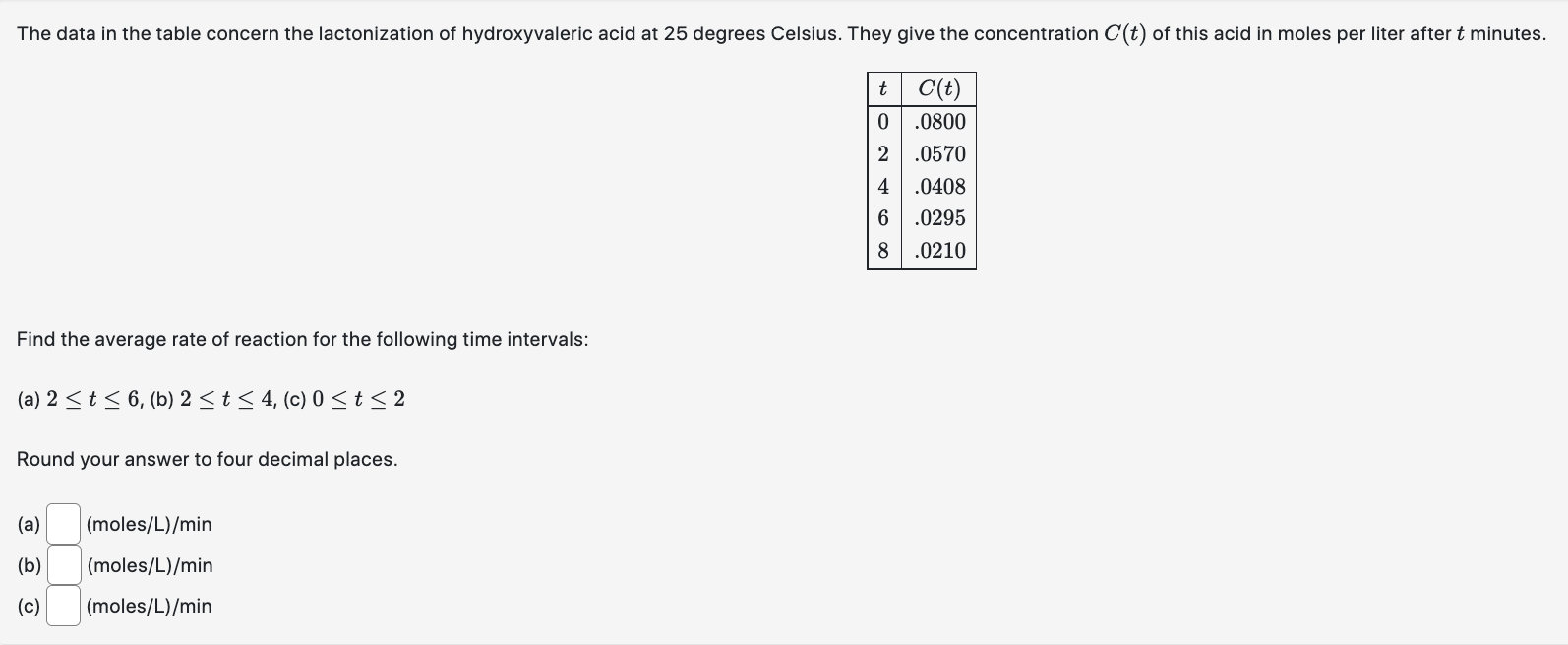



The data in the table concern the lactonization of hydroxyvaleric acid at 25 degrees Celsius. They give the concentration C(t) of this acid in moles per liter after t minutes. t C(t) 0 .0800 .0570 4 .0408 6 .0295 .0210 Find the average rate of reaction for the following time intervals: (a) 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


