Question: The data provided below (Table 1) were collected for the following reaction 904C 2NO(g) + 2H2(g) Nata) + 2H2O(a) Table 1: Experimental data for the
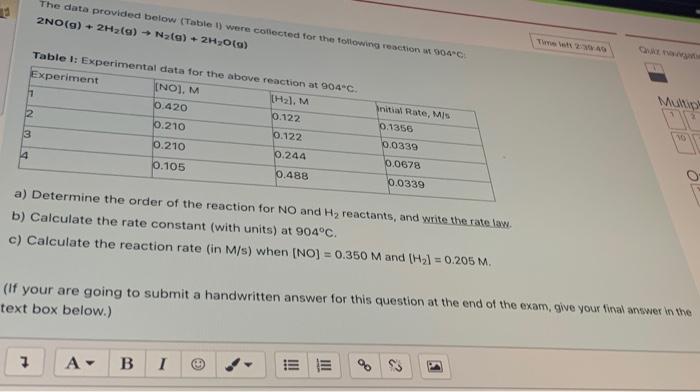
The data provided below (Table 1) were collected for the following reaction 904C 2NO(g) + 2H2(g) Nata) + 2H2O(a) Table 1: Experimental data for the above reaction at 904C. Experiment (NO), M (H2), M 11 0.420 0.122 2 0.210 0.122 3 0.210 0.244 4 0.105 0.488 Niship- 10 nitial Rate, Mis 0.1356 0.0339 0.0678 0.0339 10 a) Determine the order of the reaction for NO and Hy reactants, and write the ratew b) Calculate the rate constant (with units) at 904C. c) Calculate the reaction rate (in M/s) when (NO) = 0.350 M and (Hal = 0.205 M. (if your are going to submit a handwritten answer for this question at the end of the exam, give your walawwer the text box below.) 7 A B
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


