Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The decomposition of HI(g) is represented by the equation 2HI(g) = H(g) + I(g) The following experiment was devised to determine the equilibrium constant
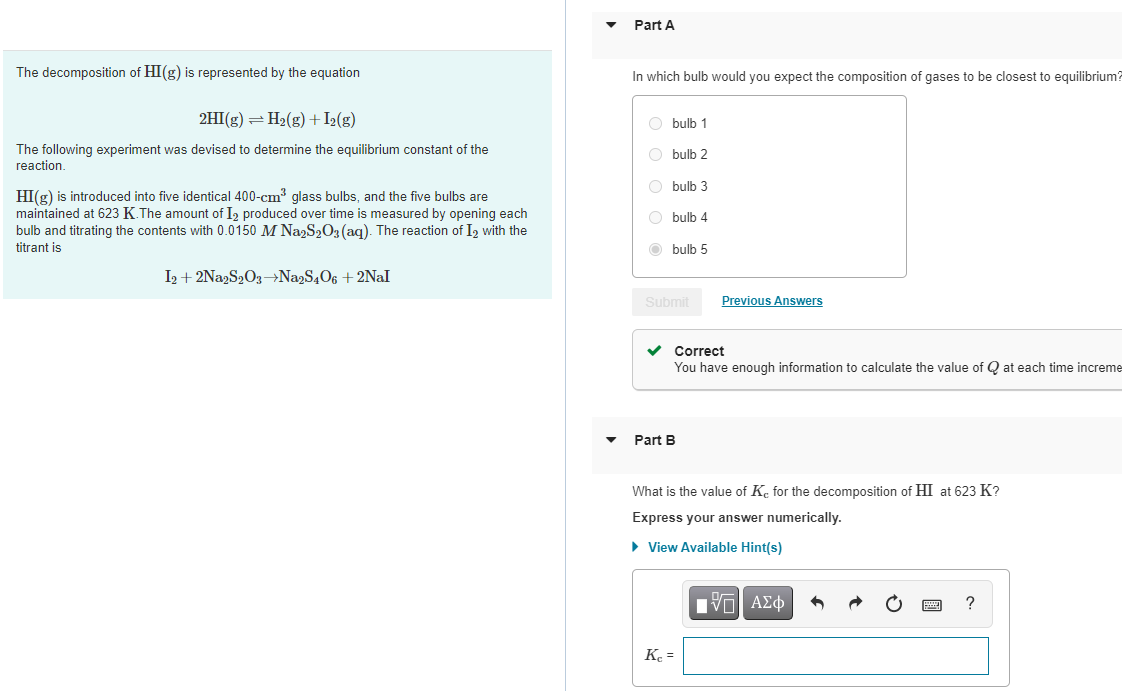
The decomposition of HI(g) is represented by the equation 2HI(g) = H(g) + I(g) The following experiment was devised to determine the equilibrium constant of the reaction. HI(g) is introduced into five identical 400-cm glass bulbs, and the five bulbs are maintained at 623 K.The amount of I produced over time is measured by opening each bulb and titrating the contents with 0.0150 M Na2SO3(aq). The reaction of I2 with the titrant is I2+2Na2S2O3-Na2S4O6 +2Nal Part A In which bulb would you expect the composition of gases to be closest to equilibrium? Obulb 1 O bulb 2 Obulb 3 O bulb 4 bulb 5 Submit Correct You have enough information to calculate the value of Q at each time increme Part B Previous Answers What is the value of K for the decomposition of HI at 623 K? Express your answer numerically. View Available Hint(s) Kc = IVE ?
Step by Step Solution
★★★★★
3.48 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


