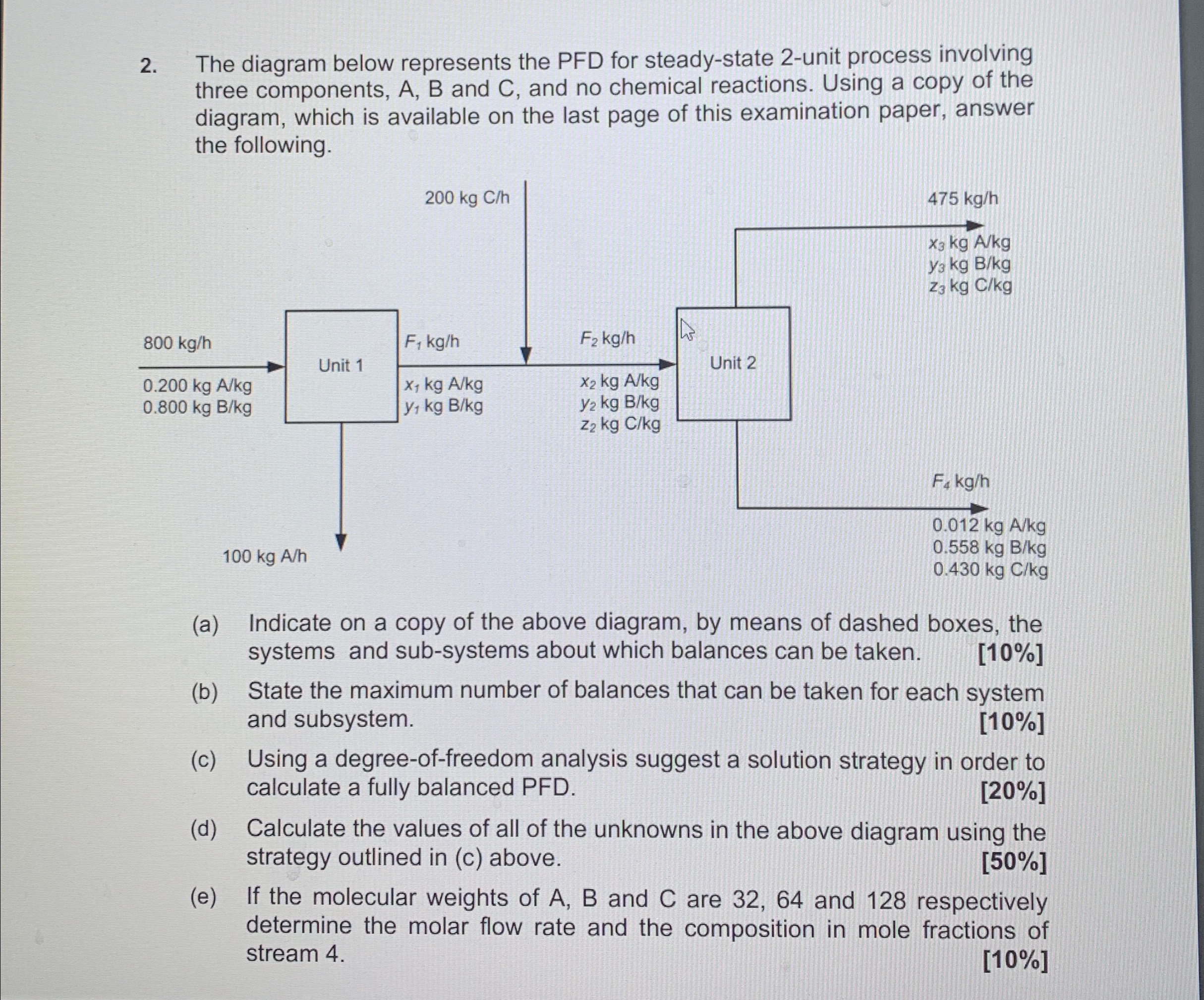
Question: The diagram below represents the PFD for steady - state 2 - unit process involving three components, A , B and C , and no
The diagram below represents the PFD for steadystate unit process involving three components, A B and C and no chemical reactions. Using a copy of the diagram, which is available on the last page of this examination paper, answer the following.
a Indicate on a copy of the above diagram, by means of dashed boxes, the systems and subsystems about which balances can be taken.
b State the maximum number of balances that can be taken for each system and subsystem.
c Using a degreeoffreedom analysis suggest a solution strategy in order to calculate a fully balanced PFD
d Calculate the values of all of the unknowns in the above diagram using the strategy outlined in c above.
e If the molecular weights of A B and C are and respectively determine the molar flow rate and the composition in mole fractions of stream
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


