Question: The element selenium would be expected to form covalent bond(s) in order to obey the octet rule. Use the octet rule to predict the formula
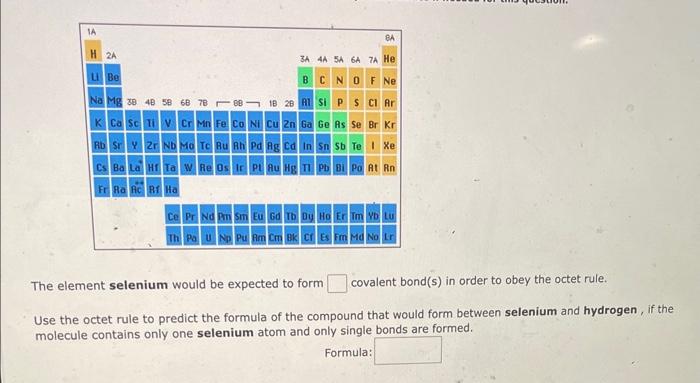
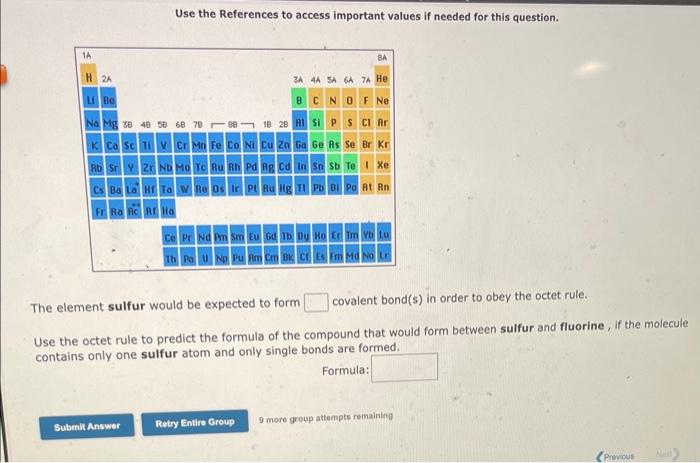
The element selenium would be expected to form covalent bond(s) in order to obey the octet rule. Use the octet rule to predict the formula of the compound that would form between selenium and hydrogen, if the molecule contains only one selenium atom and only single bonds are formed. Formula: Use the References to access important values if needed for this question. The element sulfur would be expected to form covalent bond(s) in order to obey the octet rule. Use the octet rule to predict the formula of the compound that would form between sulfur and fluorine, if the molecule contains only one sulfur atom and only single bonds are formed. Formula
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


