Question: The elementary liquid-phase reactions are carried out adiabatically in a 10 dm 3 PFR. After streams A and B mix, species A enters the reactor
The elementary liquid-phase reactions
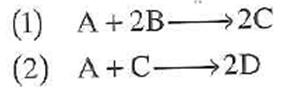
are carried out adiabatically in a 10 dm 3 PFR. After streams A and B mix, species A enters the reactor at a concentration of C A0 = 2 mol/dm 3 and species B at a concentration of 4 mol/dm 3 . The entering volumetric flow rate is 10 dm 3 /s.
Assuming you could vary the entering temperature between 300 K and 600 K, what entering temperture would you recommend to maximize the concentration of species C exiting the reactor? (±25°K). Assume all species have the same density.
Additional information
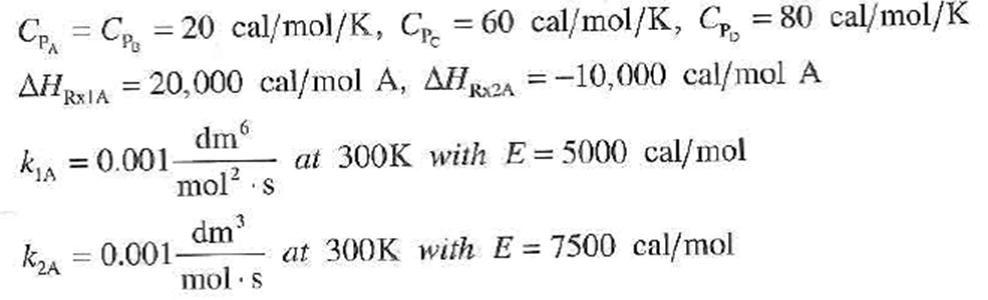
(1) A+2B-2C (2) A+C-2D C = C, = 20 cal/mol/K, C = 60 cal/mol/K, C. = 80 cal/mol/K %3D AH, RXIA = 20,000 cal/mol A, AHR2A = -10,000 cal/mol A dm %3D kA = 0.001 at 300K with E= 5000 cal/mol %3D mol?. dm k2A = 0.001- at 300K with E = 7500 cal/mol %3D mol s
Step by Step Solution
3.38 Rating (154 Votes )
There are 3 Steps involved in it
To solve this problem we need to determine the entering temperature that maximizes the concentration ... View full answer

Get step-by-step solutions from verified subject matter experts


