Question: The elementary reversible reaction A + B + 2C occurs in the liquid phase in an isothermal CSTR. A stream containing equimolar amounts of A
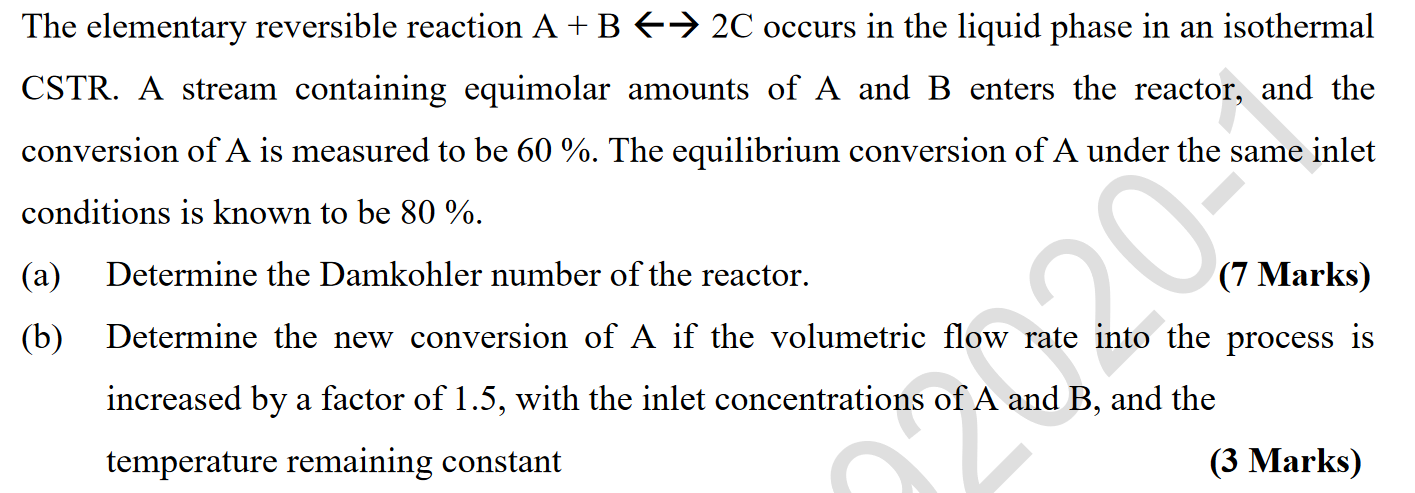
The elementary reversible reaction A + B + 2C occurs in the liquid phase in an isothermal CSTR. A stream containing equimolar amounts of A and B enters the reactor, and the conversion of A is measured to be 60 %. The equilibrium conversion of A under the same inlet conditions is known to be 80 %. (a) (b) Determine the Damkohler number of the reactor. (7 Marks) Determine the new conversion of A if the volumetric flow rate into the process is increased by a factor of 1.5, with the inlet concentrations of A and B, and the temperature remaining constant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


