Question: Problem (4). The decomposition reaction A B + C occurs in the liquid phase. It has been suggested that your company produce C from
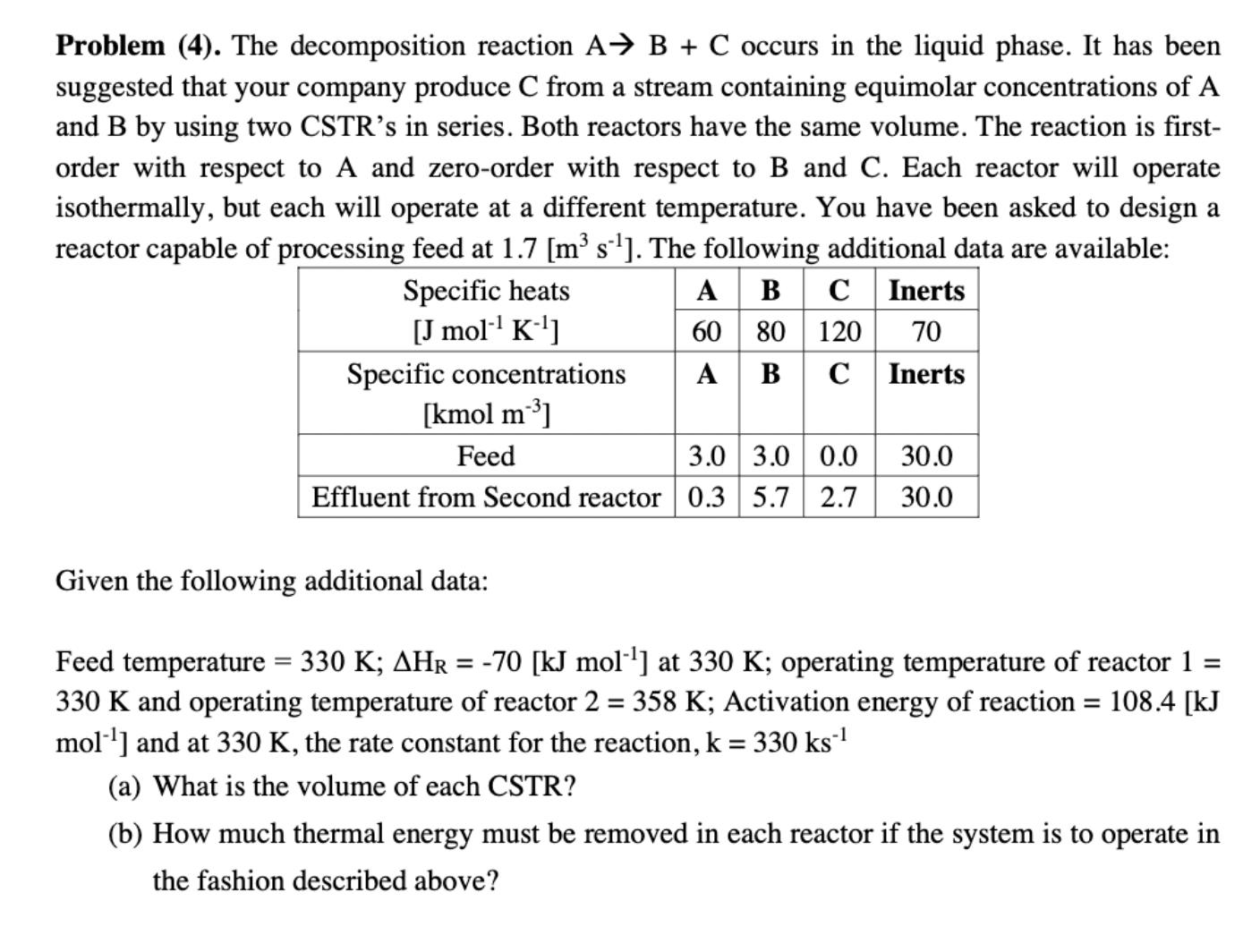
Problem (4). The decomposition reaction A B + C occurs in the liquid phase. It has been suggested that your company produce C from a stream containing equimolar concentrations of A and B by using two CSTR's in series. Both reactors have the same volume. The reaction is first- order with respect to A and zero-order with respect to B and C. Each reactor will operate isothermally, but each will operate at a different temperature. You have been asked to design a reactor capable of processing feed at 1.7 [m s]. The following additional data are available: Specific heats [J mol- K-] Specific concentrations [kmol m] ABC Inerts 60 80 120 70 AB C Inerts Feed 3.0 3.0 0.0 30.0 2.7 30.0 Effluent from Second reactor 0.35.7 Given the following additional data: = 108.4 [kJ Feed temperature = 330 K; AHR = -70 [kJ mol] at 330 K; operating temperature of reactor 1 = 330 K and operating temperature of reactor 2 = 358 K; Activation energy of reaction mol] and at 330 K, the rate constant for the reaction, k = 330 ks (a) What is the volume of each CSTR? (b) How much thermal energy must be removed in each reactor if the system is to operate in the fashion described above?
Step by Step Solution
There are 3 Steps involved in it
To determine the volume of each CSTR and the amount of thermal energy that must be removed in each reactor we need to apply the principles of reaction engineering and energy balance a Volume of each C... View full answer

Get step-by-step solutions from verified subject matter experts


