Question: The elements vanadium (V) and boron (B) form two binary compounds with the following compositions: Compound Mass % V Mass % B 1 87.606 12.394
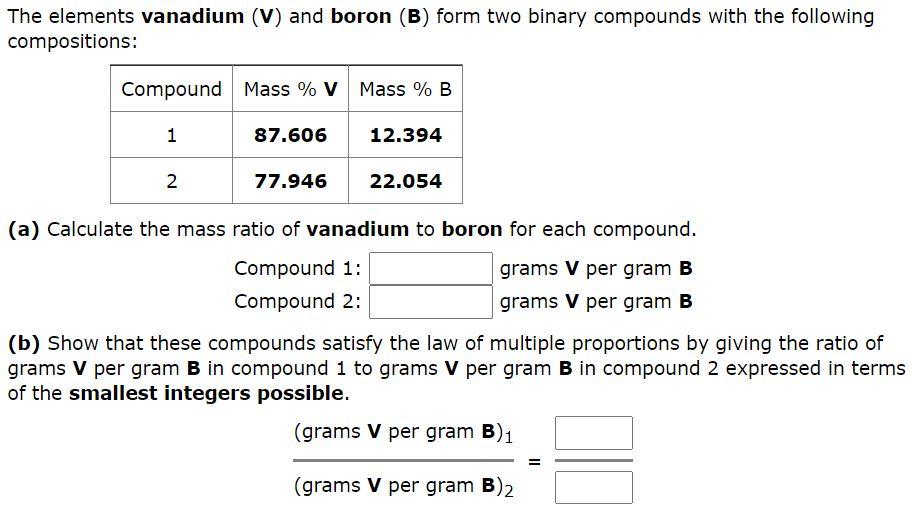
The elements vanadium (V) and boron (B) form two binary compounds with the following compositions: Compound Mass % V Mass % B 1 87.606 12.394 2 77.946 22.054 (a) Calculate the mass ratio of vanadium to boron for each compound. Compound 1: grams V per gram B Compound 2: grams V per gram B (b) Show that these compounds satisfy the law of multiple proportions by giving the ratio of grams V per gram B in compound 1 to grams V per gram B in compound 2 expressed in terms of the smallest integers possible. (grams V per gram B)1 = (grams V per gram B2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


