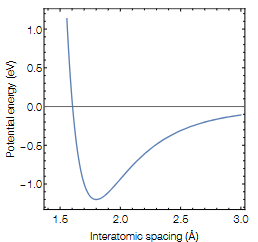
Question: The figure below shows the potential energy vs atom separation for a typical primary chemical bond. Which of the following statements are true ( mark
The figure below shows the potential energy vs atom separation for a typical primary chemical
bond. Which of the following statements are true mark all that apply
a The depth of the potential energy minimum of this curve sets the bond energy.
b The region with negative slope to the left of the minimum is repulsion arising from
core electron clouds in the two atoms resisting overlap.
c The equilibrium bond length is determined by where the curve crosses the x axis at
about
d The curvature of the potential energy around the minimum sets the bond stiffness
and is correlated with the resulting materials Youngs modulus.
e A deeper potential well correlates with lower melting temperature.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


