Question: THE FIRST ANSWER IS CORRECT. JUST NEED HELP WITH THE REST Calculate either [H3O+]or [OH]for each of the solutions at 25C. Solution A: [OH]=3.15107M;[H3O+]= M
THE FIRST ANSWER IS CORRECT. JUST NEED HELP WITH THE REST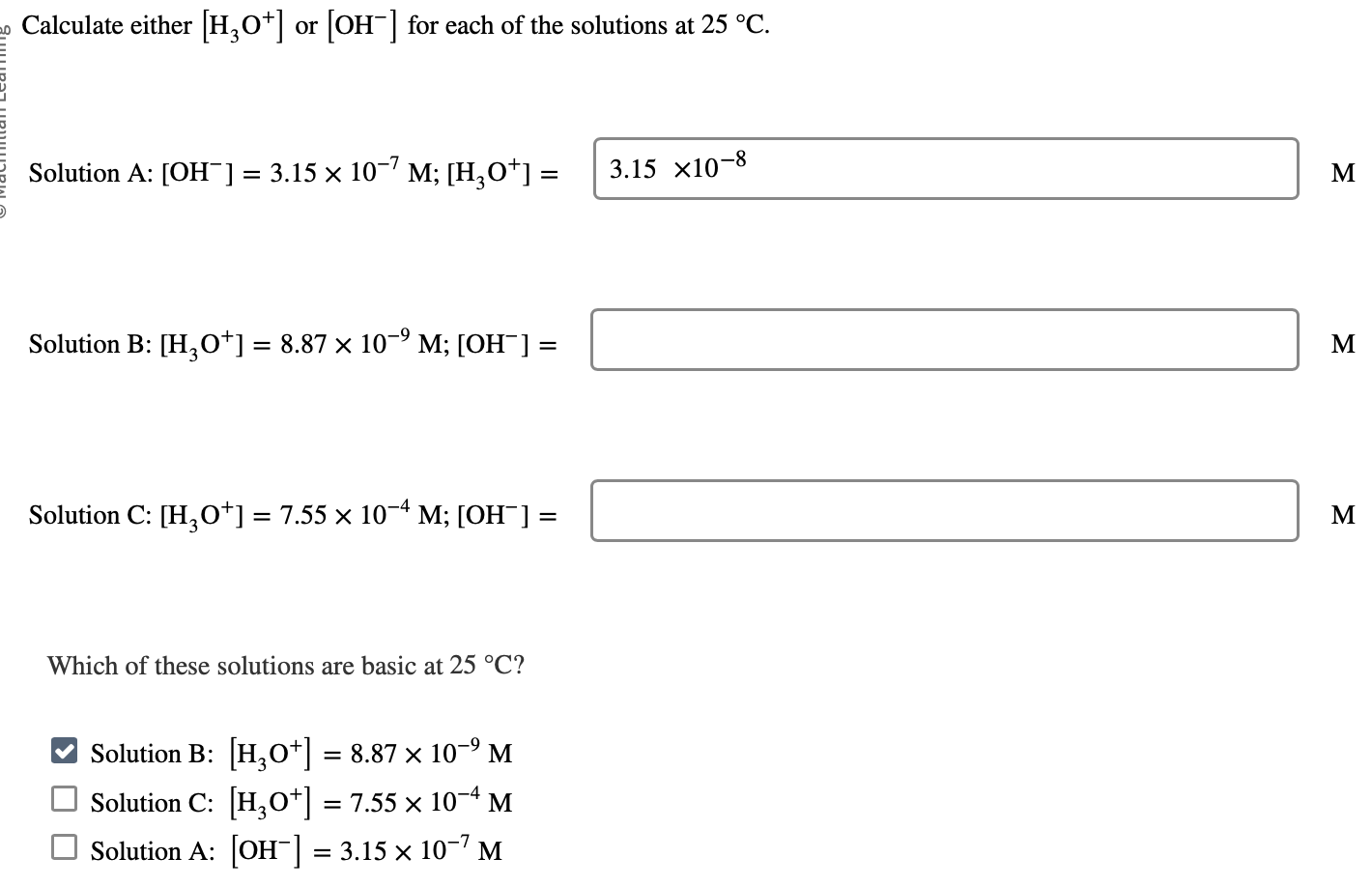
Calculate either [H3O+]or [OH]for each of the solutions at 25C. Solution A: [OH]=3.15107M;[H3O+]= M Solution B: [H3O+]=8.87109M;[OH]= M Solution C: [H3O+]=7.55104M;[OH]= M Which of these solutions are basic at 25C ? Solution B: [H3O+]=8.87109M Solution C: [H3O+]=7.55104M Solution A: [OH]=3.15107M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


