Question: The following molecular equations each show an ionic compound dissolving in water. Show the ionization process by rewriting each one as an ionic equation: a
The following molecular equations each show an ionic compound dissolving in water. Show the ionization process by rewriting each one as an ionic equation:
a
b
c
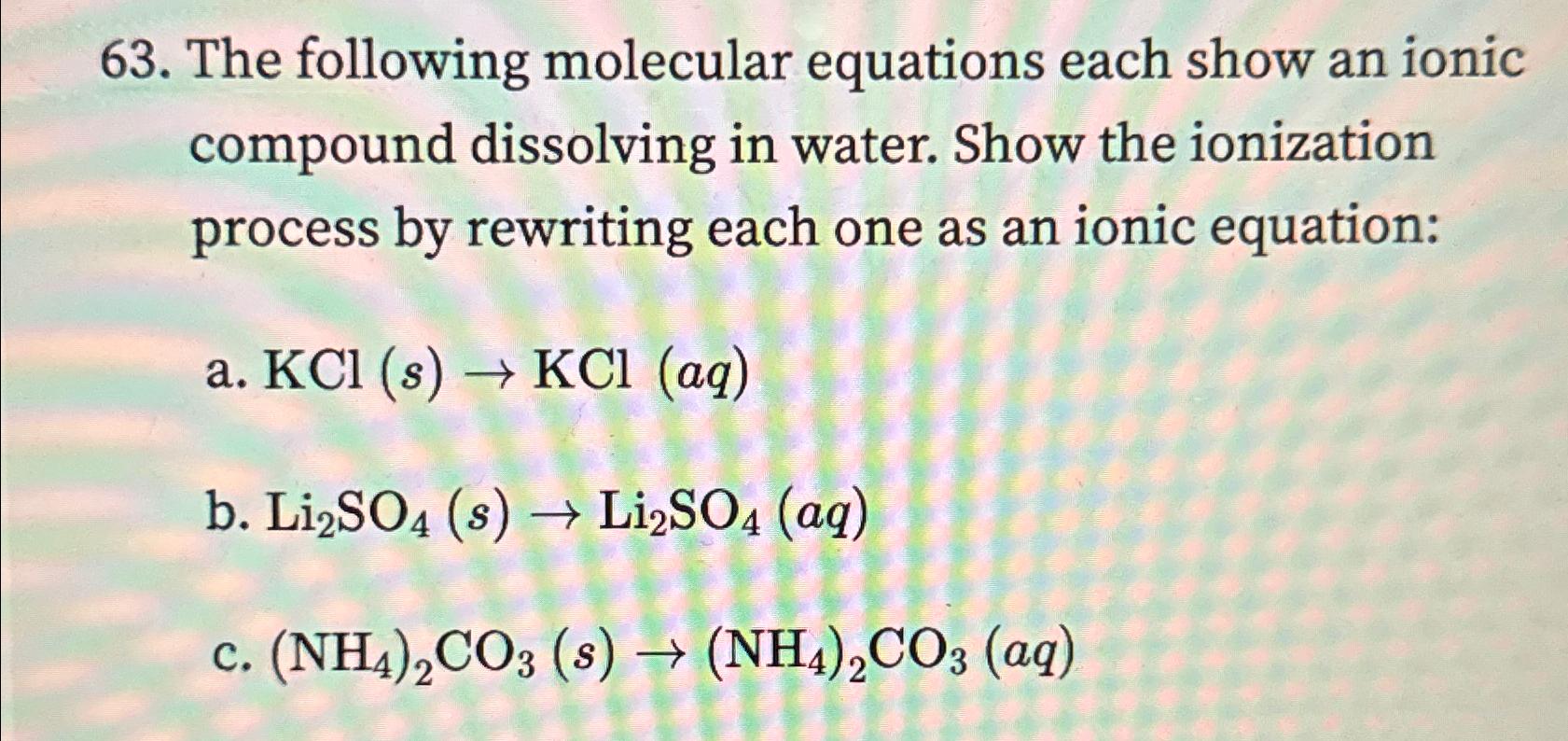
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


