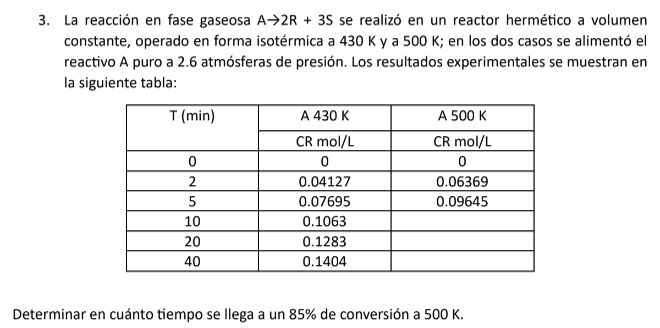
Question: The gas - phase reaction A - > 2 R + 3 S was carried out in a sealed reactor at constant volume, operated isothermally
The gasphase reaction A R S was carried out in a sealed reactor at constant volume, operated isothermally at K and K In both cases, pure reactant A was fed at atmospheres of pressure. The experimental results are shown in the following table:
Determine how long it takes to reach an conversion at K
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


