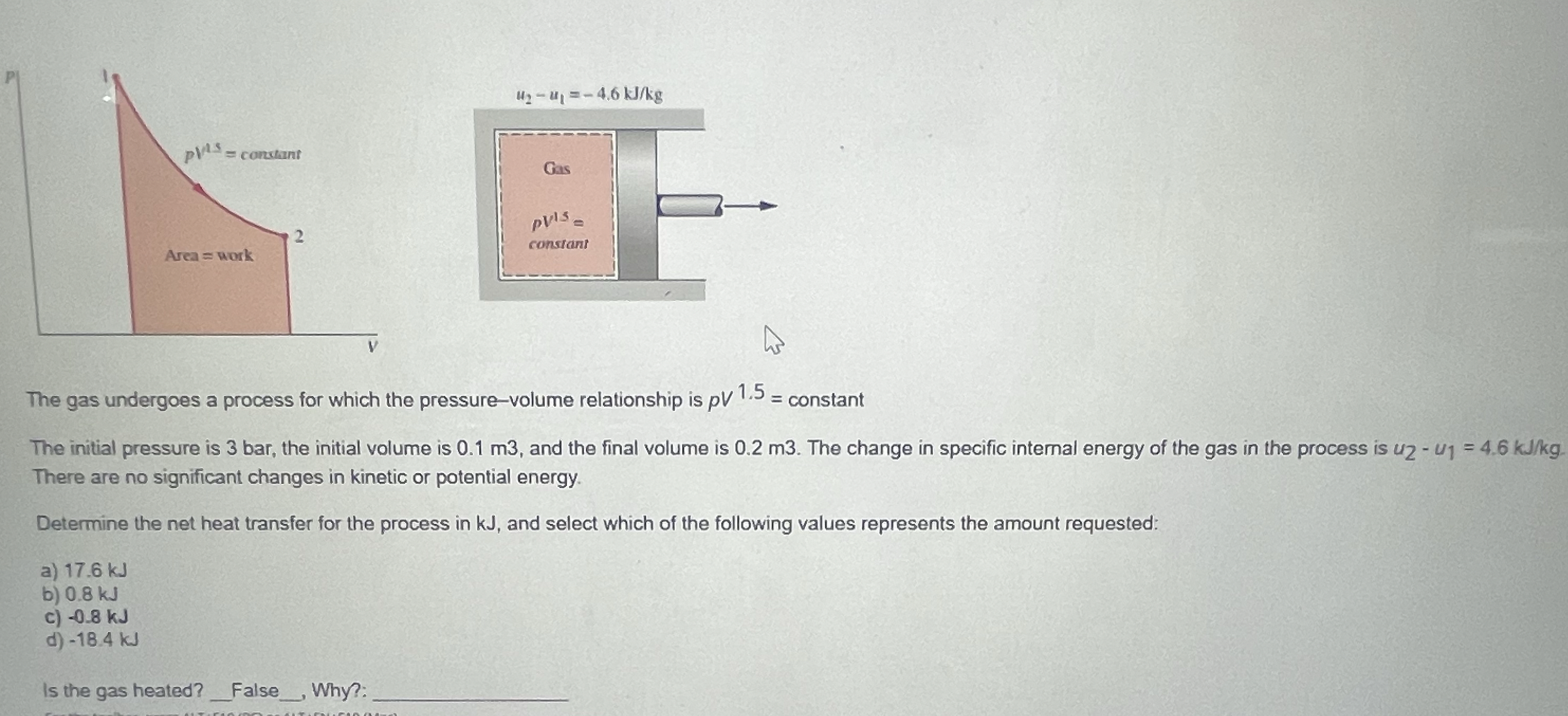
Question: The gas undergoes a process for which the pressure - volume relationship is p V 1 . 5 = constant The initial pressure is 3
The gas undergoes a process for which the pressurevolume relationship is constant
The initial pressure is bar, the initial volume is and the final volume is m The change in specific internal energy of the gas in the process is There are no significant changes in kinetic or potential energy.
Determine the net heat transfer for the process in kJ and select which of the following values represents the amount requested:
a kJ
b kJ
c kJ
d kJ
Is the gas heated? False Why?:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


