Question: The grey is the answer. Please thoroughly explain why. Thanks! 13.4 Activation Energy and Temperature Dependence of Rate Constants 8) Variation of the rate constant
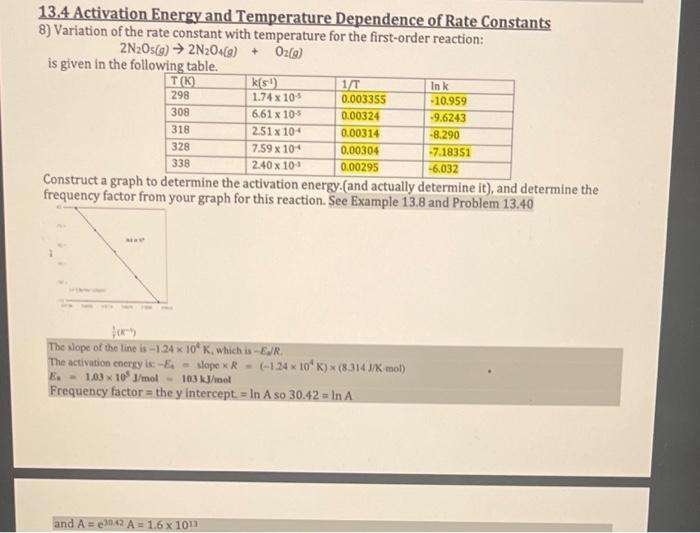
13.4 Activation Energy and Temperature Dependence of Rate Constants 8) Variation of the rate constant with temperature for the first-order reaction: 2N2O5(g)2N2O4(g)+O2(g) is given in the following table. Construct a graph to determine the activation energy.(and actually determine it), and determine the frequency factor from vour graph for this reaction. See Example 13.8 and Problem 13.40 f(x1) The slope of the line is 1.24104K, which is EJ/R. The activation coersy is E4= slope R=(124104K)(8.314J/mol) E=1.03105J/mol=103kJ/mol Frequency factor = the y intercept =lnA so 30.42=lnA
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


