Question: The initial rates of different initial reactant concentrations and at different temperatures are given the following reaction Cr(H2O)63++SCNCr(H2O)5NCS2++H2O(l) What would be the estimated activation energy
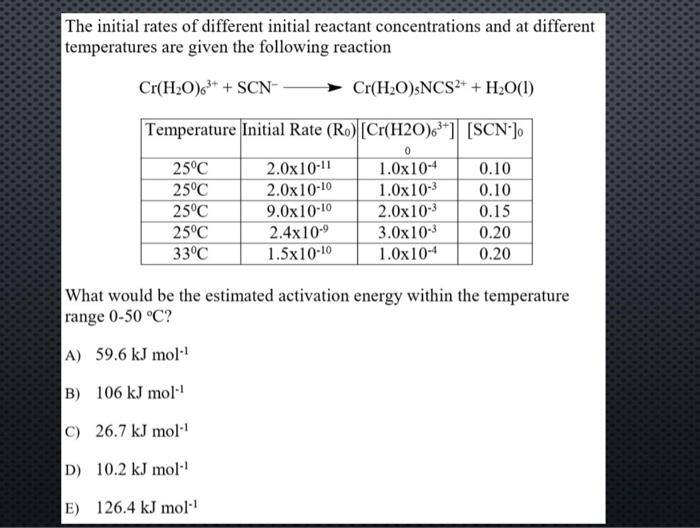
The initial rates of different initial reactant concentrations and at different temperatures are given the following reaction Cr(H2O)63++SCNCr(H2O)5NCS2++H2O(l) What would be the estimated activation energy within the temperature range 050C ? A) 59.6kJmol1 B) 106kJmol1 C) 26.7kJmol1 D) 10.2kJmol1 E) 126.4kJmol1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


