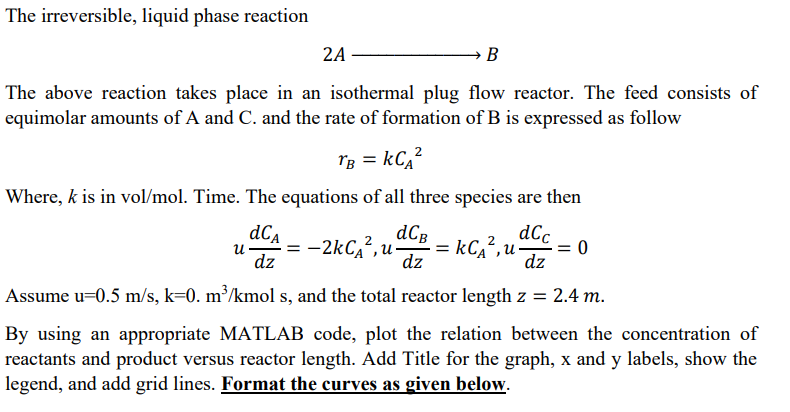
Question: The irreversible, liquid phase reaction 2 A , B The above reaction takes place in an isothermal plug flow reactor. The feed consists of equimolar
The irreversible, liquid phase reaction
The above reaction takes place in an isothermal plug flow reactor. The feed consists of
equimolar amounts of A and and the rate of formation of is expressed as follow
Where, is in Time. The equations of all three species are then
Assume mols, and the total reactor length
By using an appropriate MATLAB code, plot the relation between the concentration of
reactants and product versus reactor length. Add Title for the graph, and labels, show the
legend, and add grid lines. provide code used In Matlab
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


